What are the cost for REACH Registration
The cost of a REACH registration depends not only on the tonnage of the substance manufactured and/or imported, but also on the number of registrants wishing to register. It is possible to share the cost by sharing data through a joint submission system.
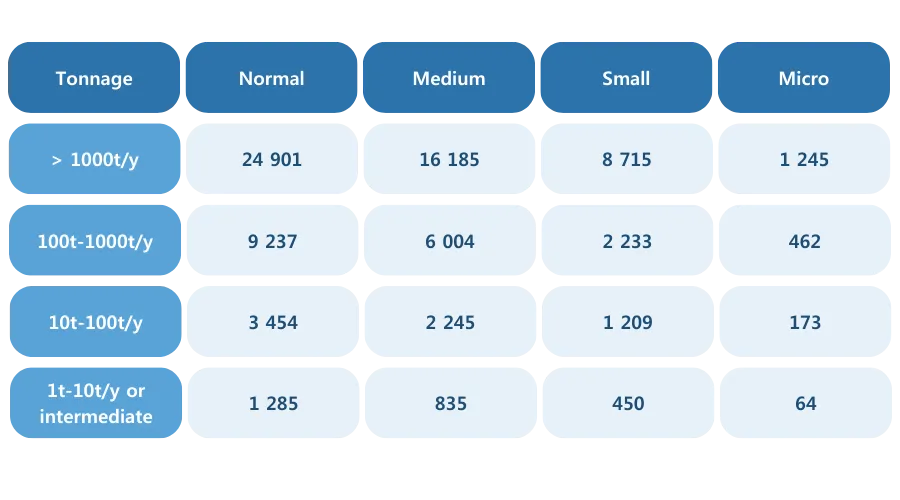
Costs associated with the REACH registration of a substance in the case of a joint submission
In the case of a joint submission, testing, preparation of the technical dossier and, in some cases, preparation of the Chemical Safety Report (CSR) are the responsibility of the lead registrant and the consortium. The latter must purchase a LoA* (Letter of Access) to enable the sharing of common registration dossier information.
Member registrants pay a fee to the lead registrant via the LoA. This fee depends on the annual tonnage of the substance and the size of the company. Small and medium-sized enterprises (SMEs) benefit from reduced ECHA fees (in euros).

Additional costs for registering a substance under REACH
To register a substance you must also take into account the fees paid to the consultants who accompany you during the registration process. This cost covers SIEF/consortium communications, data collection, preparation and submission of the IUCLID 5 dossier.
Member registrants must also carry out analyses, such as HPLC, mass spectrometry (MS), infrared (IR) and nuclear magnetic resonance (NMR), to verify the identity of the substance.
These tests can incur an additional cost of around 4,000 euros. For companies based outside Europe, the costs of OR (“Only Representative”), a status created for companies based outside the European Union, may need to be added to the joint and specific parts of the dossier.
* The Letter of Access :
1. grants permission to use and refer to one or more studies of an information holder,
2. authorizes a Member Declarant to refer to the joint submission for a certain tonnage range.
It will be useful in the event of an inspection by the competent authorities to prove compliance with the conditions set out above.The cost of the LoA can vary greatly from one joint submission to another, depending on the number of studies included in the file and the number of members who have joined the joint submission. It's important to note that you only pay for the data required for your tonnage band, not all the data included in the lead registrant's file. The cost of the LoA also depends on the tonnage band. EcoMundo can help you identify the LoA costs for the substances you intend to register.
Please note! There are different ways of calculating the LoA, depending on whether cost-sharing is based on :
- The assumed number of registrants
- The evolution of the number of registrants
- In all cases, the calculation method must comply with the rules set out in Regulation 2016/9 published in January 2016.



