Approved suppliers

What is an approved supplier under the European Biocides Regulations?
From 1 September 2015, a biocidal product (PB) cannot be placed on the European market if the manufacturer or importer of the active substance (AS), or if the importer of PB himself, is not included in the official list of approved suppliers (Article 95 of the Biocidal Product Regulation).
For suppliers outside Europe, it is possible to call for a European representative to be included in the list.
The regulations of biocidal products are intended to:
- Promote data sharing avoiding duplicating the same tests on vertebrate animals. The Agency itself regulates its tests and requires verification of similar tests before starting new ones in the framework of the review program.
- Eliminate all “free-riders”, marketing a BP without having participated in the evaluation costs of the dossier on the active substances that it contains.
Where to find information about the list of approved suppliers?
You can access the updated list of approved suppliers by clicking here.
The procedure to be included in the list
Two scenarios are available to you. Inclusion in this list is automatic for:
- The participants in the review program
- Any filing of an application file for a new AS
Here is the list of automatic inclusions.
If you are not a part of this list, you should file the following documents via the R4PB potal:
- A complete file in accordance with the annex II of the RPB
OR
- A reference to a dossier already submitted and for which the period of data protection has been exceeded (10 years for an existing AS or 15 years for a new AS)
OR - A letter of Access (LoA) for a completed AS file already submitted
This last option is the most common, it concerns including any company wishing to date to comply with the requirements of Article 95.
Steps to follow for the inclusion in the list of approved suppliers
By communicating in your supply chain, the goal is to identify if any of your suppliers is approved and included in the list.
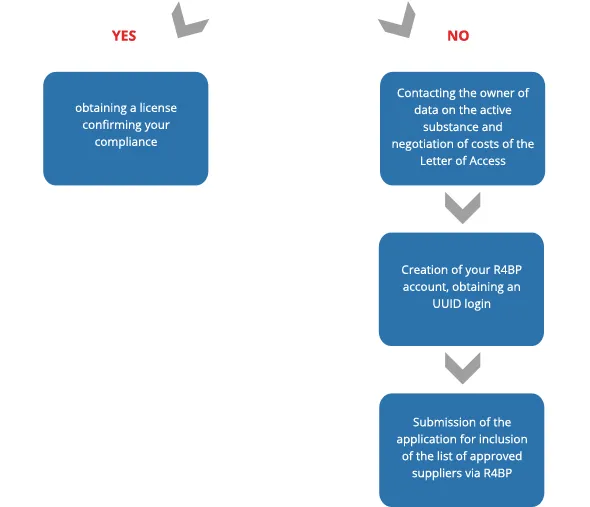
Once your application for inclusion is submitted, you will appear on ECHA's provisional list during the evaluation time of your dossier.
Cases not affected by this measure
You are not affected by this measure if:
- You are an active substances supplier or importer listed in the Annex I of BPR, in categories 1 to 5 and 7, or biocidal products containing just those substances. The substances in the Annex I are considered non hazardous, such as lavender oil or citronella.
- You are an AS importer or supplier in biocidal products concerned by the BPR but which were not under the BPD.
Any biocidal product on the market must satisfy other regulatory requirements. To learn more, do not hesistate to read our blog post on the biocides cheklist
-





