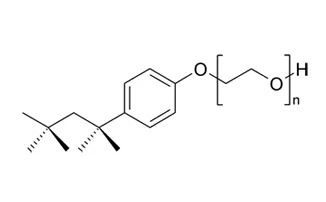
Identity of 4-Nonylphenol, branched and linear, ethoxylated
Name Triton or 4-Nonylphenol, branched and linear, ethoxylated
IUPAC name 4-Nonylphenol, branched and linear, ethoxylated [substances with a linear and/or branched alkyl chain with a carbon number of 9 covalently bound in position 4 to phenol, ethoxylated covering UVCB- and well-defined substances, polymers and homologues, which include any of the individual isomers and/or combinations thereof]
CAS number - EC number -
Formule chimique C15H24O
Hazard classification of 4-Nonylphenol, branched and linear, ethoxylated
CORROSIVE
● H314: Causes severe skin burns and eye damage
HAZARDOUS TO THE AQUATIC ENVIRONMENT
● H400 : Very toxic to aquatic life
● H412 : Harmful to aquatic life with long lasting effects
VERY HAZARDOUS TO HEALTH
● H350: May cause cancer
● H340: May cause genetic defects
● H360: May damage fertility or the unborn child
● H371: May cause damage to organs
● H304: May be fatal if swallowed and enters airways
● H334: May cause allergy or asthma symptoms or breathing difficulties if inhaled
HAZARDOUS TO THE OZONE LAYER
● EUH 059: Hazardous to the ozone layer
4-Nonylphenol, branched and linear, ethoxylated in Annex XIV of REACH
Key regulatory information
Intrinsic property for which it was included in Annex XIV of REACH endocrine disrupting properties [article 57, point f) — environment]
Sunset date 4 January 2021
Latest date to submit the authorisation dossier to ECHA 4 July 2019
Who is concerned by REACH authorisation? Main industries concerned Pharmaceutical, chemical, paint, metallurgy, agriculture
EcoMundo’s advice to succeed in your authorisation application
Recommendations for your authorisation dossier
- The substance entered Annex XIV of REACH for its endocrine disrupting properties.
- In addition to the CSR and Analysis of Alternatives, the applicant will have to conduct a socio-economic analysis for the dossier.
- Whether the substance works with a threshold or not is not determined for these endocrine disruptors.
- This means that the chosen route for the creation of the dossier is not set in stone. The European Commission specifies that if an applicant decides to take the risk management route, it will be the applicant’s responsibility to demonstrate the existence of threshold effects and their level. The RAC will then assess this demonstration.
- Whichever authorisation route chosen, "risk management" or "socio-economic analysis" (SEA), EcoMundo recommends to create an SEA dossier in case the relevance of the threshold demonstration is questioned by the committee assessors.
- Another challenge could be the possible exemption of the activity involving "Scientific Research and Development" activities, particularly for the Pharmaceutical and In Vitro diagnostic industries.
- To assess the impacts for health and the environment, EcoMundo recommends to double the approaches through modelling and data monitoring
- Key elements of a successful authorisation dossier:
- A specific request with the most elements possible in relation to your activity
- Transparence and realism of the hypotheses and data as all elements produced will be challenged by the committees or stakeholders
- Underlining the exposure for man and the environment with associated emission measures
- The choice of the monetary assessment methodology is key for this dossier
Planning recommendations
- The Latest Application date for this substance is 4 July 2019, the last submission window is on May 2019.
- The creation of a dossier is usually between 6 and 18 months depending on the complexity of the dossier (intrinsic properties of the substance, complexity of the applicants’ value and production chain.
- It is therefore recommended to start the creation of the dossier immediately (september 2017).
Identity of 4-Nonylphenol, branched and linear, ethoxylated
Name Triton or 4-Nonylphenol, branched and linear, ethoxylated
IUPAC name 4-Nonylphenol, branched and linear, ethoxylated [substances with a linear and/or branched alkyl chain with a carbon number of 9 covalently bound in position 4 to phenol, ethoxylated covering UVCB- and well-defined substances, polymers and homologues, which include any of the individual isomers and/or combinations thereof]
CAS number - EC number -
Formule chimique C15H24O
Hazard classification of 4-Nonylphenol, branched and linear, ethoxylated
CORROSIVE
● H314: Causes severe skin burns and eye damage
HAZARDOUS TO THE AQUATIC ENVIRONMENT
● H400 : Very toxic to aquatic life
● H412 : Harmful to aquatic life with long lasting effects
VERY HAZARDOUS TO HEALTH
● H350: May cause cancer
● H340: May cause genetic defects
● H360: May damage fertility or the unborn child
● H371: May cause damage to organs
● H304: May be fatal if swallowed and enters airways
● H334: May cause allergy or asthma symptoms or breathing difficulties if inhaled
HAZARDOUS TO THE OZONE LAYER
● EUH 059: Hazardous to the ozone layer
4-Nonylphenol, branched and linear, ethoxylated in Annex XIV of REACH
Key regulatory information
Intrinsic property for which it was included in Annex XIV of REACH endocrine disrupting properties [article 57, point f) — environment]
Sunset date 4 January 2021
Latest date to submit the authorisation dossier to ECHA 4 July 2019
Who is concerned by REACH authorisation? Main industries concerned Pharmaceutical, chemical, paint, metallurgy, agriculture
EcoMundo’s advice to succeed in your authorisation application
Recommendations for your authorisation dossier
- The substance entered Annex XIV of REACH for its endocrine disrupting properties.
- In addition to the CSR and Analysis of Alternatives, the applicant will have to conduct a socio-economic analysis for the dossier.
- Whether the substance works with a threshold or not is not determined for these endocrine disruptors.
- This means that the chosen route for the creation of the dossier is not set in stone. The European Commission specifies that if an applicant decides to take the risk management route, it will be the applicant’s responsibility to demonstrate the existence of threshold effects and their level. The RAC will then assess this demonstration.
- Whichever authorisation route chosen, "risk management" or "socio-economic analysis" (SEA), EcoMundo recommends to create an SEA dossier in case the relevance of the threshold demonstration is questioned by the committee assessors.
- Another challenge could be the possible exemption of the activity involving "Scientific Research and Development" activities, particularly for the Pharmaceutical and In Vitro diagnostic industries.
- To assess the impacts for health and the environment, EcoMundo recommends to double the approaches through modelling and data monitoring
- Key elements of a successful authorisation dossier:
- A specific request with the most elements possible in relation to your activity
- Transparence and realism of the hypotheses and data as all elements produced will be challenged by the committees or stakeholders
- Underlining the exposure for man and the environment with associated emission measures
- The choice of the monetary assessment methodology is key for this dossier
Planning recommendations
- The Latest Application date for this substance is 4 July 2019, the last submission window is on May 2019.
- The creation of a dossier is usually between 6 and 18 months depending on the complexity of the dossier (intrinsic properties of the substance, complexity of the applicants’ value and production chain.
- It is therefore recommended to start the creation of the dossier immediately (september 2017).







