ISO 29621: Risk assessment and identification of microbiologically low-risk products in cosmetics

Definition
Low risk products are described in the guideline as: any “product whose environment denies microorganisms the physical and chemical requirements for growth and/or survival”.
The guideline specifies 3 cases:
- “This category of low-risk products applies to microbiological contamination which may occur during manufacturing and/or intended use by the consumer.”
- “A product whose packaging prevents the ingress of microorganisms is considered a microbiological low-risk product during its use”.
- "The inclusion of preservatives or other antimicrobial compounds in a formulation by itself would not necessarily constitute a low-risk product.”
Scope of application
The ISO 29621:2017 guidance covers finished cosmetic products that, based on a risk assessment, are presenting a low risk of microbial contamination during production and/or intended use.
Identified products in the guideline do not require the application of microbiological International standards for Cosmetics commonly called “challenge test” or ISO 11930.
Risk assessment factor
Some products present the ideal conditions for microbial contamination and growth of microorganisms. Indeed, water, low or high PH value, or nutrients can lead to a microbial risk. Hence, advanced testing as stability test and challenge test are required to ensure the safety of your cosmetic products.
Products based on alcohol or low water activity are considered as low risk products regarding a potential microbiological contamination.
Identified Low risk products
Here is a non-exhaustive list of products with a low risk of microbial growth due to the physico-chemical characteristics:
- Water activity of formulation : aw, < or = 0.75
- pH of formulation : < or = 3 and = or > 10%
- Alcohol content: if > or = to 20%
- Production conditions: filing temperature > 65°C
- Packaging: single-use products and products which cannot be opened
- Raw materials that can create a hostile environment:
- Polar organic solvents
- Oxidizing dyes
- Propellant gases
- Aluminium chlorohydrate and related salts: > 25%
- Strong oxidizing agent or strong reducing agents
- Other substance if supported by data
Note: In this case a strong documentation will be asked to create the PIF
- Combined factors: combined factor mentioned in the guideline can create an environment that is hostile to microbial growth or survival. This exemption requires evidence and is the responsibility of the manufacturer.
Example of low risk products
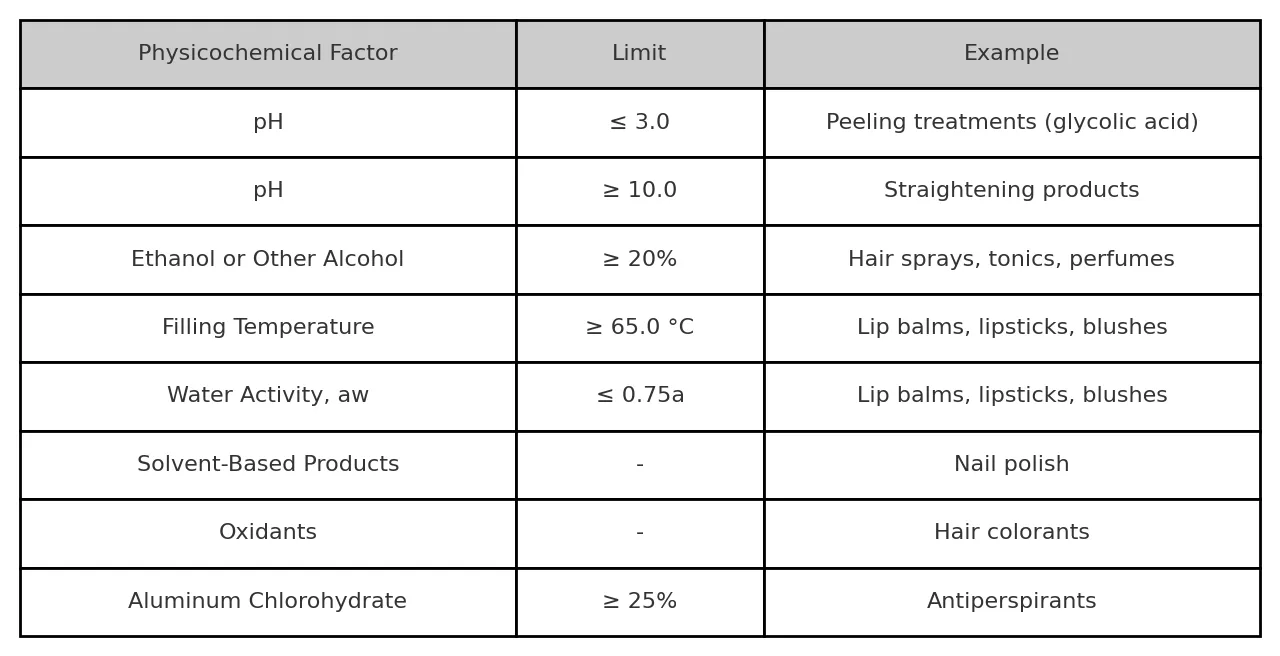
Source: ISO 29621:2017
Note: Exemption from challenge testing and the use of this guideline must be based on appropriate justification and supported by reliable data.
The ISO Standard car be purchased on the ISO website.
Definition
Low risk products are described in the guideline as: any “product whose environment denies microorganisms the physical and chemical requirements for growth and/or survival”.
The guideline specifies 3 cases:
- “This category of low-risk products applies to microbiological contamination which may occur during manufacturing and/or intended use by the consumer.”
- “A product whose packaging prevents the ingress of microorganisms is considered a microbiological low-risk product during its use”.
- "The inclusion of preservatives or other antimicrobial compounds in a formulation by itself would not necessarily constitute a low-risk product.”
Scope of application
The ISO 29621:2017 guidance covers finished cosmetic products that, based on a risk assessment, are presenting a low risk of microbial contamination during production and/or intended use.
Identified products in the guideline do not require the application of microbiological International standards for Cosmetics commonly called “challenge test” or ISO 11930.
Risk assessment factor
Some products present the ideal conditions for microbial contamination and growth of microorganisms. Indeed, water, low or high PH value, or nutrients can lead to a microbial risk. Hence, advanced testing as stability test and challenge test are required to ensure the safety of your cosmetic products.
Products based on alcohol or low water activity are considered as low risk products regarding a potential microbiological contamination.
Identified Low risk products
Here is a non-exhaustive list of products with a low risk of microbial growth due to the physico-chemical characteristics:
- Water activity of formulation : aw, < or = 0.75
- pH of formulation : < or = 3 and = or > 10%
- Alcohol content: if > or = to 20%
- Production conditions: filing temperature > 65°C
- Packaging: single-use products and products which cannot be opened
- Raw materials that can create a hostile environment:
- Polar organic solvents
- Oxidizing dyes
- Propellant gases
- Aluminium chlorohydrate and related salts: > 25%
- Strong oxidizing agent or strong reducing agents
- Other substance if supported by data
Note: In this case a strong documentation will be asked to create the PIF
- Combined factors: combined factor mentioned in the guideline can create an environment that is hostile to microbial growth or survival. This exemption requires evidence and is the responsibility of the manufacturer.
Example of low risk products

Source: ISO 29621:2017
Note: Exemption from challenge testing and the use of this guideline must be based on appropriate justification and supported by reliable data.
The ISO Standard car be purchased on the ISO website.




.png)


