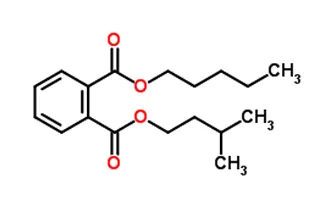
N-pentyl-isopentylphthalate was included in REACH Annex XIV on June 14, 2017. It will be completely banned on European soil from January 4, 2019, unless you apply for REACH authorization. What do you need to know before applying? Our experts give you all the key facts about this substance, as well as some advice to keep in mind before taking the plunge.
Identité du N-pentyl-isopentylphtalate
Name: N-pentyl-isopentylphtalate
IUPAC Name: N-pentyl-isopentylphtalate
CAS Number: 776297-69-9
Chemical formula: C18H26O4
Hazard classification of N-pentyl-isopentylphtalate

VERY HAZARDOUS TO HEALTH
● H350: May cause cancer
● H340: May cause genetic defects
● H360: May damage fertility or the unborn child
● H371: May cause damage to organs
● H304: May be fatal if swallowed and enters airways

HAZARDOUS TO THE AQUATIC ENVIRONMENT
● H400: Very toxic to aquatic life
N-pentyl-isopentylphtalate in annex XIV of REACH
Intrinsic property for which it was included in Annex XIV of REACH
Toxic for reproduction (category 1B)
Sunset date 4 July 2020
Latest date to submit the authorisation dossier to ECHA 4 January 2019
Who is concerned by REACH authorisation? Main industries concerned Polymers
EcoMundo’s advice to succeed in your authorisation application
Recommendations for your authorisation dossier
- As the substance does not have a threshold, the Socio-Economic Analysis route will be chosen. This analysis will demonstrate that the benefits related to the granting of the authorisation outweigh the risks associated.
- The applicant will thus have to conduct a socio-economic analysis as well as the CSR and Analysis of Alternatives.
- The preferred methodology to conduct the socio-economic analysis of the authorisation application is the cost/benefit analysis.
- The creation of a dossier is usually between 6 and 18 months depending on the complexity of the dossier (intrinsic properties of the substance, complexity of the applicants’ value and production chain.
- Key elements of a successful authorisation dossier:
- A specific request with the most elements possible in relation to your activity
- Transparence and realism of the hypotheses and data as all elements produced will be challenged by the committees or stakeholders
- The “applied for use” and “non-use” scenarios are essential for the relevance of the socio-economic analysis
Planning recommendations
- The Latest Application date for this substance is 04/01/2019, the last submission window in on November 2018.
- It is therefore recommended to start the creation of the dossier immediately (september 2017).
N-pentyl-isopentylphthalate was included in REACH Annex XIV on June 14, 2017. It will be completely banned on European soil from January 4, 2019, unless you apply for REACH authorization. What do you need to know before applying? Our experts give you all the key facts about this substance, as well as some advice to keep in mind before taking the plunge.
Identité du N-pentyl-isopentylphtalate
Name: N-pentyl-isopentylphtalate
IUPAC Name: N-pentyl-isopentylphtalate
CAS Number: 776297-69-9
Chemical formula: C18H26O4
Hazard classification of N-pentyl-isopentylphtalate

VERY HAZARDOUS TO HEALTH
● H350: May cause cancer
● H340: May cause genetic defects
● H360: May damage fertility or the unborn child
● H371: May cause damage to organs
● H304: May be fatal if swallowed and enters airways

HAZARDOUS TO THE AQUATIC ENVIRONMENT
● H400: Very toxic to aquatic life
N-pentyl-isopentylphtalate in annex XIV of REACH
Intrinsic property for which it was included in Annex XIV of REACH
Toxic for reproduction (category 1B)
Sunset date 4 July 2020
Latest date to submit the authorisation dossier to ECHA 4 January 2019
Who is concerned by REACH authorisation? Main industries concerned Polymers
EcoMundo’s advice to succeed in your authorisation application
Recommendations for your authorisation dossier
- As the substance does not have a threshold, the Socio-Economic Analysis route will be chosen. This analysis will demonstrate that the benefits related to the granting of the authorisation outweigh the risks associated.
- The applicant will thus have to conduct a socio-economic analysis as well as the CSR and Analysis of Alternatives.
- The preferred methodology to conduct the socio-economic analysis of the authorisation application is the cost/benefit analysis.
- The creation of a dossier is usually between 6 and 18 months depending on the complexity of the dossier (intrinsic properties of the substance, complexity of the applicants’ value and production chain.
- Key elements of a successful authorisation dossier:
- A specific request with the most elements possible in relation to your activity
- Transparence and realism of the hypotheses and data as all elements produced will be challenged by the committees or stakeholders
- The “applied for use” and “non-use” scenarios are essential for the relevance of the socio-economic analysis
Planning recommendations
- The Latest Application date for this substance is 04/01/2019, the last submission window in on November 2018.
- It is therefore recommended to start the creation of the dossier immediately (september 2017).







