
The BPC’s work programme
The active substances listed are those for which the competent authority of the assessment has confirmed to SECR (ECHA secretariat) that it was preparing the necessary documents for the assessment during the meetings planned in the programme.
The substances are presented in a table, next to the type of product in which it is found, the competent authority in charge of its assessment, if the substance is candidate to substitution according to the competent authority report, if the substance meets the exclusion criteria according to the CAR.
You can read through the BPC’s work programme on ECHA’s website.
The last opinions published concerned Margosa extract for PT 19 and Fludioxonil for PT 7, 9 and 10.
Reminder of the product-types considered:
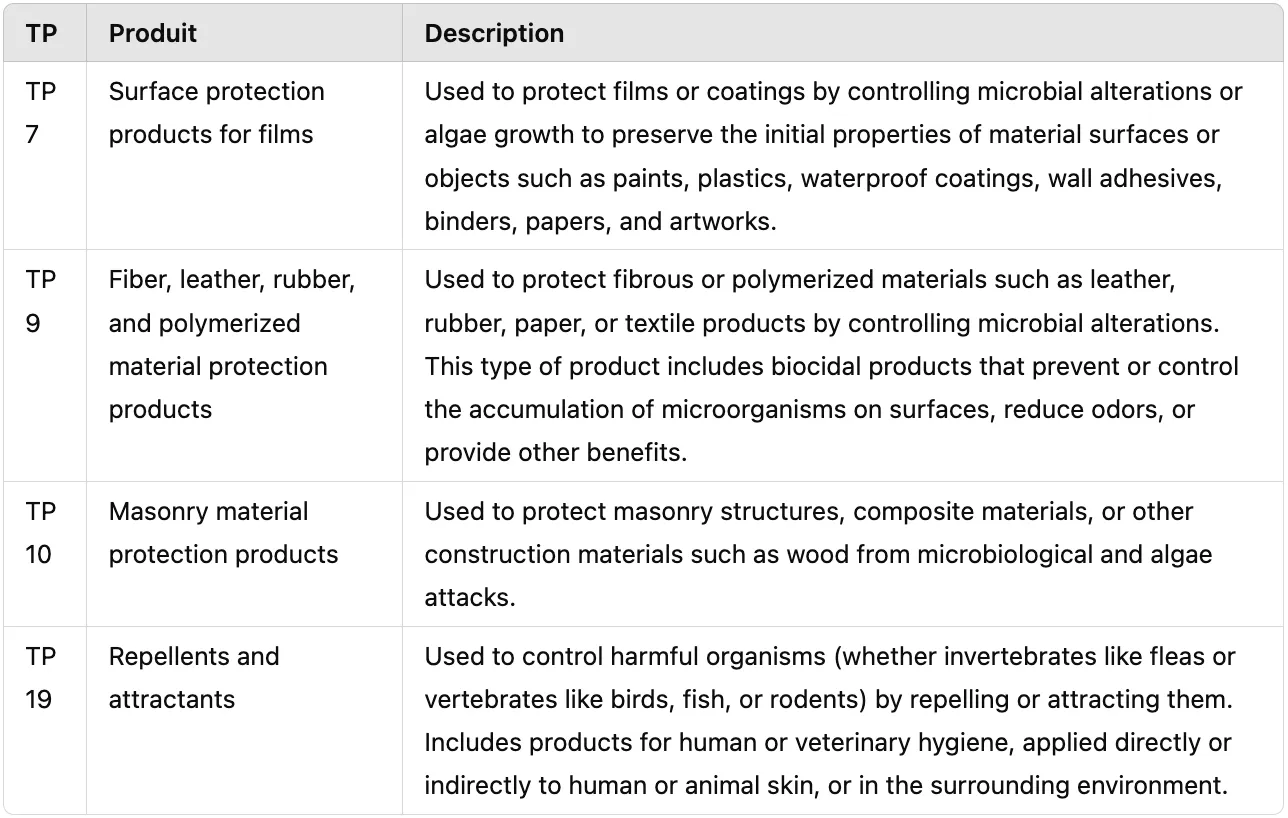
source: ECHA
The 4 opinions adopted by the BPC
Margosa extract for PT 19
Context
Margosa extract is oil made from the cold press extraction of Azadirachta indica seeds with the help of carbon dioxide.
Use
Margosa extract is used to control harmful organisms (invertebrates such as fleas, vertebrates such as birds, fish, rodents), by repelling or attracting, including those that are used for human or veterinary hygiene either directly on the skin or indirectly in the environment of humans or animals.
Decision
Exposure risks for human health relating to a use against harmful organisms has been deemed “acceptable”.
Exposure risks for the environment required an additional risk assessment, based on tonnage, to take into account the unequal spatial and temporal distributions for surface water, sediment, and soil. These risks are deemed acceptables. As Margosa extract does not meet the criteria to be classified as a CMR (Carcinogenic, mutagenic and toxic to reproduction), PBT (Persistant, Bioaccumulative and Toxic), vPvB (very Persistant and very Bioaccumulative), endocrine disruptor or respiratory sensitizer, there have not been any concerns relating to its critical effects, or to the amount non-active isomers.
Its use is deemed safe for human health as well as for the environment. It is not considered as a candidate to substitution.
Fluodoxinol for PT 7,9 and 10
Context
Its real name, 4-(2,2-difluoro-1,3-benzodioxol-4-yl)-1H- pyrrole-3-carbonitrile was evaluated for PT 7,9 and 10.
Use
Fluodoxinol is used:
- for the preservation of films or coatings by the control of microbial deterioration or algal growth in order to protect the initial properties of the surface of materials or objects such as paints, plastics, sealants, wall adhesives, binders, papers, art works.
- for the preservation of fibrous or polymerised materials, such as leather, rubber or paper or textile products by the control of microbiological deterioration. This product-type includes biocidal products which antagonise the settlement of micro-organisms on the surface of materials and therefore hamper or prevent the development of odour and/or offer other kinds of benefits.
- for the preservation of masonry, composite materials, or other construction materials other than wood by the control of microbiological and algal attack.
Decision
(Valid for 3 product types: PT7, PT9, PT10)
Exposure risks for human health and the environment relating to a use against harmful organisms has been deemed “acceptable”. Its use has been deemed safe for human health and for the environment. Fluodoxinol does not meet the criteria to be classified as a CMR (Carcinogenic, mutagenic and toxic to reproduction), PBT (Persistant, Bioaccumulative and Toxic), vPvB (very Persistant and very Bioaccumulative), endocrine disruptor or respiratory sensitizer. There have not been any concerns relating to its critical effects, or to the amount non-active isomers.
Substances under evaluation
Cypermethrin, used for the control of arthropods (such as insects, arachnids and crustaceans PT 18)), as well as MIT (5-chloro-2-methyl-2H-isothiazol-3-un) used as a slimicide (PT12) are under evaluation by the committee.
You wish to learn more?
EcoMundo’s experts help you:
- Establish a global strategy for your dossier creation.
- Identify the size of your company and confirm it with the Agency to receive a discount for SMEs
- Create the « Data gap » from the tox and ecotox data to provide (read-across strategy, QSAR…)
- With the scientific argumentation
- Collect relevant data: analytical, efficacy, toxicology, ecotoxicology, residue anaysis, etc.
- Fill out the dossier in IUCLID 6 format and generate the dossier
- Submit the dossier via R4BP
The BPC’s work programme
The active substances listed are those for which the competent authority of the assessment has confirmed to SECR (ECHA secretariat) that it was preparing the necessary documents for the assessment during the meetings planned in the programme.
The substances are presented in a table, next to the type of product in which it is found, the competent authority in charge of its assessment, if the substance is candidate to substitution according to the competent authority report, if the substance meets the exclusion criteria according to the CAR.
You can read through the BPC’s work programme on ECHA’s website.
The last opinions published concerned Margosa extract for PT 19 and Fludioxonil for PT 7, 9 and 10.
Reminder of the product-types considered:

source: ECHA
The 4 opinions adopted by the BPC
Margosa extract for PT 19
Context
Margosa extract is oil made from the cold press extraction of Azadirachta indica seeds with the help of carbon dioxide.
Use
Margosa extract is used to control harmful organisms (invertebrates such as fleas, vertebrates such as birds, fish, rodents), by repelling or attracting, including those that are used for human or veterinary hygiene either directly on the skin or indirectly in the environment of humans or animals.
Decision
Exposure risks for human health relating to a use against harmful organisms has been deemed “acceptable”.
Exposure risks for the environment required an additional risk assessment, based on tonnage, to take into account the unequal spatial and temporal distributions for surface water, sediment, and soil. These risks are deemed acceptables. As Margosa extract does not meet the criteria to be classified as a CMR (Carcinogenic, mutagenic and toxic to reproduction), PBT (Persistant, Bioaccumulative and Toxic), vPvB (very Persistant and very Bioaccumulative), endocrine disruptor or respiratory sensitizer, there have not been any concerns relating to its critical effects, or to the amount non-active isomers.
Its use is deemed safe for human health as well as for the environment. It is not considered as a candidate to substitution.
Fluodoxinol for PT 7,9 and 10
Context
Its real name, 4-(2,2-difluoro-1,3-benzodioxol-4-yl)-1H- pyrrole-3-carbonitrile was evaluated for PT 7,9 and 10.
Use
Fluodoxinol is used:
- for the preservation of films or coatings by the control of microbial deterioration or algal growth in order to protect the initial properties of the surface of materials or objects such as paints, plastics, sealants, wall adhesives, binders, papers, art works.
- for the preservation of fibrous or polymerised materials, such as leather, rubber or paper or textile products by the control of microbiological deterioration. This product-type includes biocidal products which antagonise the settlement of micro-organisms on the surface of materials and therefore hamper or prevent the development of odour and/or offer other kinds of benefits.
- for the preservation of masonry, composite materials, or other construction materials other than wood by the control of microbiological and algal attack.
Decision
(Valid for 3 product types: PT7, PT9, PT10)
Exposure risks for human health and the environment relating to a use against harmful organisms has been deemed “acceptable”. Its use has been deemed safe for human health and for the environment. Fluodoxinol does not meet the criteria to be classified as a CMR (Carcinogenic, mutagenic and toxic to reproduction), PBT (Persistant, Bioaccumulative and Toxic), vPvB (very Persistant and very Bioaccumulative), endocrine disruptor or respiratory sensitizer. There have not been any concerns relating to its critical effects, or to the amount non-active isomers.
Substances under evaluation
Cypermethrin, used for the control of arthropods (such as insects, arachnids and crustaceans PT 18)), as well as MIT (5-chloro-2-methyl-2H-isothiazol-3-un) used as a slimicide (PT12) are under evaluation by the committee.
You wish to learn more?
EcoMundo’s experts help you:
- Establish a global strategy for your dossier creation.
- Identify the size of your company and confirm it with the Agency to receive a discount for SMEs
- Create the « Data gap » from the tox and ecotox data to provide (read-across strategy, QSAR…)
- With the scientific argumentation
- Collect relevant data: analytical, efficacy, toxicology, ecotoxicology, residue anaysis, etc.
- Fill out the dossier in IUCLID 6 format and generate the dossier
- Submit the dossier via R4BP







