.png)
Regulatory Amendment for Cosmetics in Canada
On April 24, 2024, Health Canada announced a significant update concerning the disclosure of ingredients in cosmetics, aligning standards with those of the European Union. This change affects both existing and new products on the Canadian market.
Our team of experts at EcoMundo has analyzed these new regulatory updates to provide you with a clear and practical summary of the upcoming requirements. Discover the key steps to ensure your cosmetic products comply with this revised regulation.
New labeling requirements for cosmetics in Canada
In response to Health Canada's update, any company selling cosmetic products in Canada that contain fragrance allergens must now comply with strict new disclosure standards. These regulations, aligned with European Union directives, require particular attention to allergen concentrations, depending on whether the product is to be rinsed off or left on the skin.
New definitions
With this update, key definitions in the cosmetics industry have been clarified:
📖 INCI name: means the International Nomenclature Cosmetic Ingredient name assigned to an ingredient in the International Cosmetic Ingredient Dictionary and Handbook published by the Personal Care Products Council on its website, as amended from time to time.
🏷️ Inner label: means a label on or affixed to the immediate container of a cosmetic.
🏭 Manufacturer: means a person, a partnership or an unincorporated association that sells, or manufactures and sells, a cosmetic under its own name or under a trademark, design, trade name or other name or mark owned or controlled by it.
🌍 Importer: means a person who imports a cosmetic into Canada for the purpose of selling it.
🚿 rinse-off product: means any cosmetic that is intended to be removed after application to the skin, hair or mucous membranes.
💧 leave-on product: means any cosmetic that is intended to stay in prolonged contact with the skin, hair or mucous membranes.
Labeling requirements for cosmetics
Requirements for contact information on cosmetic inner labels have been adjusted.
The regulations stipulate that all cosmetic products must be notified to Health Canada within ten days of their first sale in the country, or of any modification to the product. This notification must include:
- Contact information on the product's inner label.
- The function of the cosmetic, specifying whether it is a rinse-off or leave-on product.
- List of ingredients by INCI name.
- The name and Canadian address of the manufacturer or importer.
All required information on the label must appear in both French and English.
It is forbidden to sell a cosmetic unless its internal label contains a telephone number, email address, website address, postal address or any other information allowing communication with a contact person to whom consumers can address their questions about the cosmetic.
Need help notifying your products?
Contact our experts now, they'll be happy to help you!

Labeling standards for fragrance allergens
Updated regulations require precise labeling of fragrance allergens in cosmetics, according to their concentration.
- For rinse-off products, any fragrance allergen present in excess of 0.01% must be clearly indicated on the label.
- For leave-on products, the threshold is even lower, at 0.001%.
This requirement is in line with European Union directives, and the list of allergens to be declared is based on Annex III of European Cosmetics Regulation 1223/2009.
Labeling flexibility for small packages
Specific options have been established for the labeling of cosmetic products in small packages. When packaging is too small to include all the required information, manufacturers can opt for:
(a) The addition of on a tag, tape or card affixed to the container or package where ingredients can be listed.
(b) Publication of the ingredient list on a website. In this case, the product's external label must clearly state that the list of ingredients is available online and must include the location of the website.
Learn more about cosmetic product labeling in Canada?
> Check out our article on what should appear on cosmetic products labels in Canada.
Notification and safety of cosmetic products in Canada
When notifying Health Canada of cosmetic products within ten days of their first sale, it is mandatory to include complete information on the product, its manufacturer or importer.
Updates to the form require specifying whether the product is rinse-off or not, declaring fragrance allergens, and adjusting ingredient concentration ranges. It is no longer necessary to list distributors, but a Canadian address must be included.
In the event of non-compliance or failure to provide proof of safety within the specified deadlines, the sale of the product must be suspended immediately.
Mandatory notification:
📅 Deadline: Notification to Health Canada within 10 days of sales.
📝 Content: Detailed information about the product and its manufacturer or importer.
Proof of Safety:
📜 Official Request: The Minister may require written proof of a product's safety.
🕒 Due Date: Within 10 days after the day of the Minister�s request.
🔍 Conformity: Need to prove safety under normal or recommended conditions of use.
In the event of Non-Compliance:
❌ Action: Sale of product suspended immediately if evidence not sufficient or not provided.
⏳ Delay: Suspension takes effect the day after the deadline for submission of evidence.
Want to learn more about cosmetic regulations in Canada?
> Read our article on understanding Canada's cosmetic regulations.
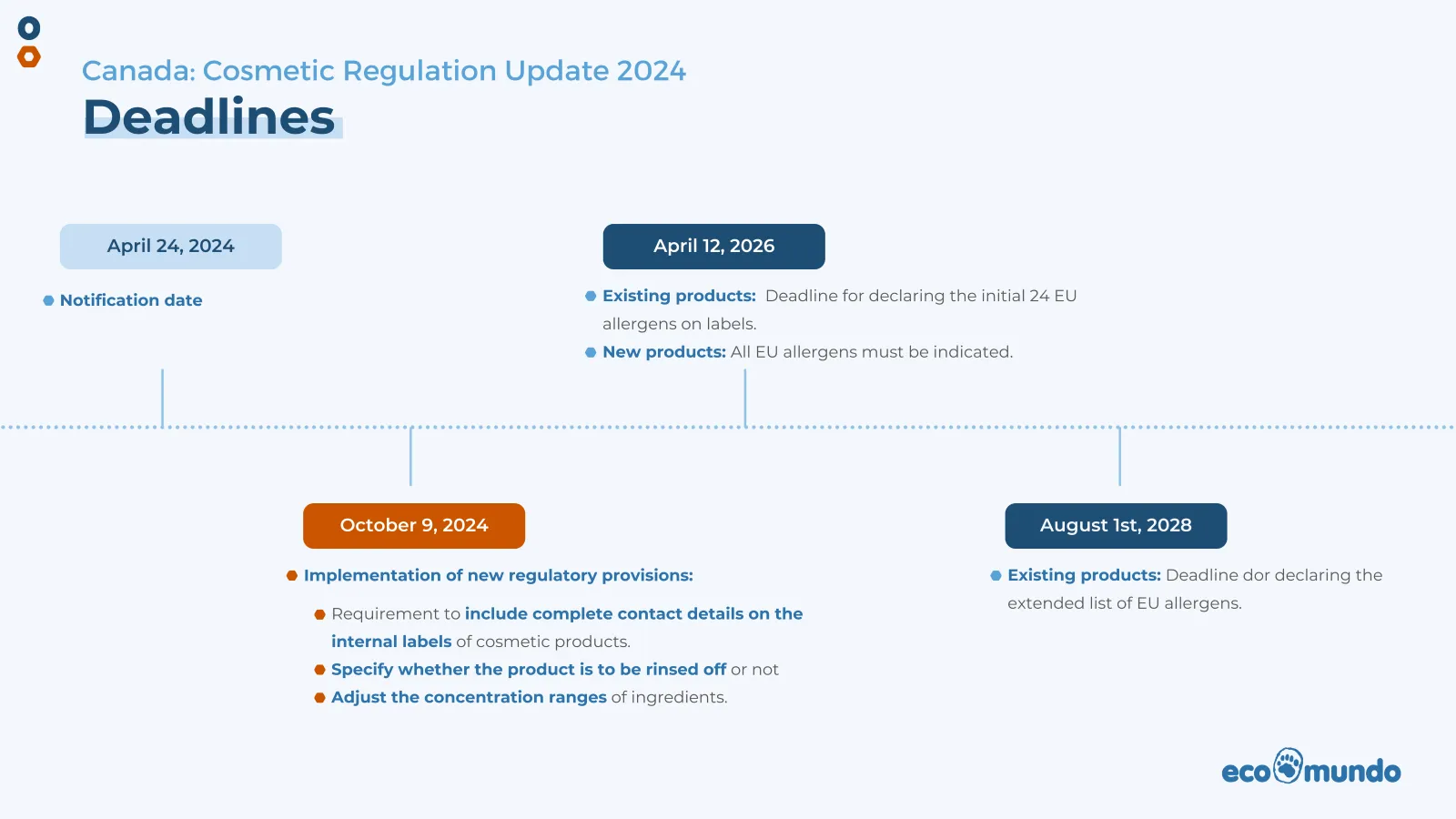
Regulatory deadlines for cosmetic products in Canada
Octobre 9, 2024
- All new regulations come into force, with the exception of allergen labeling, which has an extended deadline.
April 12, 2026
- Existing products: Deadline to include the initial 24 EU allergens on labels.
- New products: All EU allergens (initial 24 plus extended list) must be indicated as soon as they are introduced on the market.
August 1st, 2028
- Existing products: Deadline for inclusion of the extended list of EU allergens on labels.
How to prepare for the new cosmetic law standards in Canada?
Prepare the information you need for an effective declaration. Keep at hand ingredient concentrations, product descriptions and contacts for any requests for additional information.
For more information, contact EcoMundo's experts, who are on hand to help you navigate these regulatory changes and identify products in your portfolio that may be affected.
Regulatory Amendment for Cosmetics in Canada
On April 24, 2024, Health Canada announced a significant update concerning the disclosure of ingredients in cosmetics, aligning standards with those of the European Union. This change affects both existing and new products on the Canadian market.
Our team of experts at EcoMundo has analyzed these new regulatory updates to provide you with a clear and practical summary of the upcoming requirements. Discover the key steps to ensure your cosmetic products comply with this revised regulation.
New labeling requirements for cosmetics in Canada
In response to Health Canada's update, any company selling cosmetic products in Canada that contain fragrance allergens must now comply with strict new disclosure standards. These regulations, aligned with European Union directives, require particular attention to allergen concentrations, depending on whether the product is to be rinsed off or left on the skin.
New definitions
With this update, key definitions in the cosmetics industry have been clarified:
📖 INCI name: means the International Nomenclature Cosmetic Ingredient name assigned to an ingredient in the International Cosmetic Ingredient Dictionary and Handbook published by the Personal Care Products Council on its website, as amended from time to time.
🏷️ Inner label: means a label on or affixed to the immediate container of a cosmetic.
🏭 Manufacturer: means a person, a partnership or an unincorporated association that sells, or manufactures and sells, a cosmetic under its own name or under a trademark, design, trade name or other name or mark owned or controlled by it.
🌍 Importer: means a person who imports a cosmetic into Canada for the purpose of selling it.
🚿 rinse-off product: means any cosmetic that is intended to be removed after application to the skin, hair or mucous membranes.
💧 leave-on product: means any cosmetic that is intended to stay in prolonged contact with the skin, hair or mucous membranes.
Labeling requirements for cosmetics
Requirements for contact information on cosmetic inner labels have been adjusted.
The regulations stipulate that all cosmetic products must be notified to Health Canada within ten days of their first sale in the country, or of any modification to the product. This notification must include:
- Contact information on the product's inner label.
- The function of the cosmetic, specifying whether it is a rinse-off or leave-on product.
- List of ingredients by INCI name.
- The name and Canadian address of the manufacturer or importer.
All required information on the label must appear in both French and English.
It is forbidden to sell a cosmetic unless its internal label contains a telephone number, email address, website address, postal address or any other information allowing communication with a contact person to whom consumers can address their questions about the cosmetic.
Need help notifying your products?
Contact our experts now, they'll be happy to help you!

Labeling standards for fragrance allergens
Updated regulations require precise labeling of fragrance allergens in cosmetics, according to their concentration.
- For rinse-off products, any fragrance allergen present in excess of 0.01% must be clearly indicated on the label.
- For leave-on products, the threshold is even lower, at 0.001%.
This requirement is in line with European Union directives, and the list of allergens to be declared is based on Annex III of European Cosmetics Regulation 1223/2009.
Labeling flexibility for small packages
Specific options have been established for the labeling of cosmetic products in small packages. When packaging is too small to include all the required information, manufacturers can opt for:
(a) The addition of on a tag, tape or card affixed to the container or package where ingredients can be listed.
(b) Publication of the ingredient list on a website. In this case, the product's external label must clearly state that the list of ingredients is available online and must include the location of the website.
Learn more about cosmetic product labeling in Canada?
> Check out our article on what should appear on cosmetic products labels in Canada.
Notification and safety of cosmetic products in Canada
When notifying Health Canada of cosmetic products within ten days of their first sale, it is mandatory to include complete information on the product, its manufacturer or importer.
Updates to the form require specifying whether the product is rinse-off or not, declaring fragrance allergens, and adjusting ingredient concentration ranges. It is no longer necessary to list distributors, but a Canadian address must be included.
In the event of non-compliance or failure to provide proof of safety within the specified deadlines, the sale of the product must be suspended immediately.
Mandatory notification:
📅 Deadline: Notification to Health Canada within 10 days of sales.
📝 Content: Detailed information about the product and its manufacturer or importer.
Proof of Safety:
📜 Official Request: The Minister may require written proof of a product's safety.
🕒 Due Date: Within 10 days after the day of the Minister�s request.
🔍 Conformity: Need to prove safety under normal or recommended conditions of use.
In the event of Non-Compliance:
❌ Action: Sale of product suspended immediately if evidence not sufficient or not provided.
⏳ Delay: Suspension takes effect the day after the deadline for submission of evidence.
Want to learn more about cosmetic regulations in Canada?
> Read our article on understanding Canada's cosmetic regulations.
Regulatory deadlines for cosmetic products in Canada
Octobre 9, 2024
- All new regulations come into force, with the exception of allergen labeling, which has an extended deadline.
April 12, 2026
- Existing products: Deadline to include the initial 24 EU allergens on labels.
- New products: All EU allergens (initial 24 plus extended list) must be indicated as soon as they are introduced on the market.
August 1st, 2028
- Existing products: Deadline for inclusion of the extended list of EU allergens on labels.

How to prepare for the new cosmetic law standards in Canada?
Prepare the information you need for an effective declaration. Keep at hand ingredient concentrations, product descriptions and contacts for any requests for additional information.
For more information, contact EcoMundo's experts, who are on hand to help you navigate these regulatory changes and identify products in your portfolio that may be affected.




.png)


