
The Safe Cosmetics Act versus the EU Cosmetic Regulation
To better understand labeling requirements, let’s start with a bit of context on labeling, packaging and marketing of cosmetic products.
U.S. Regulation
You will usually find two types of laws in the United States: Federal and local regulation. We will focus on Federal laws, and will only mention the most popular local regulations: Proposition 65 in California, California Safety Act, Chemical of High Concern to Children in Washington State, Part I of Chapter 499 of Florida statutes, Wholesale Food & Cosmetic Project in New Jersey.
Cosmetic products are regulated by the Food and Drugs Administration (FDA) through different laws:
- Federal Food Drugs and Cosmetic Act (FD&C) of 1938, as amended
- Fair Packaging and Labeling Act
In 2010, the “Safe Cosmetics Act” gave the FDA power to control chemical substances in cosmetics and the possibility to ban carcinogens and endocrine disruptors. The FD&C Act prohibits the marketing of adulterated or misbranded cosmetics in interstate commerce. A new amendment was scheduled for 2012, and supported by the Republican Leonard Lance.
The Food and Drugs Administration is also competent for color additives. They set up guidelines for manufacturers to assess the safety of their own products:
- Hazards Identification
- Evaluation of the dose-response relationship
- Exposure assessment
- Risk Characterization
To achieve these goals, companies can rely on:
- Available toxicological data tests on similar ingredients and other product formulation
- If necessary, the realization of additional toxicological tests regarding available information and data.
For the moment, there is no regulation related to nanomaterials.
The FD&C Act also includes a reporting program named “Voluntary Cosmetic Registration Program”- (VCRP) for the manufacturers. The VCRP is divided in two parts:
- Registration of companies
- Cosmetic Product Ingredient Statement
If you don’t know how to categorize your product you can read this guide: Is my product a Cosmetic, a drug, or both? (Or is it Soap?)
The EU Cosmetic Regulation
The EU Cosmetic Regulation 1223/2009 came into force in 2013 and concerns 31 European countries (28 countries of the EU + Norway + Iceland + Lichtenstein). The regulation is based on three principles:
- Safety of Raw Materials and Ingredients
- Good manufacturing practices
- Invigilating of cosmetic market
These principles translate into requirements for the cosmetic brand (non-exhaustive list):
- Designate a Responsible Person (RP)
- Prepare a Product Information file (PIF) including a Safety Assessment
- Respect the Good manufacturing practices (GMP) for cosmetics
- Comply with Labeling and Packaging requirements
- Ensure notification via the Cosmetic Products Notification Portal (CPNP)
With this regulation, the European Union sets a higher level of transparency for finished cosmetic products, prevents the placing on the market of hazardous substances, and strengthen safety for consumers.
Labeling requirements: US versus EU
U.S. labeling requirements
The cosmetics distributed in the US must comply with the Cosmetic Labeling Guide published by the FDA under the authority of the FD&C Act. Labeling means all labels and other written, printed or graphic matter on or accompanying a product.
The main requirements are:
Principal Display Panel (PDP)
- Identity statement: The common or usual name, descriptive name, fanciful name, Illustration, Prominence, Placement
- Net quantity: Quantity of the contents, in terms of weight, measure, numerical count
- Warning: If the cosmetic product contains an ingredient for which adequate substantiation of safety has not been obtained, a warning must be placed on the PDP like “Warning - the safety of this product has not been determined”
Please note: The label statements required under the authority of the FD&C Act must appear on the inside as well as any outside container or wrapper.
Information panel (IP):
- Language: All label or labeling statements required by law or regulation must be in English. If the label contains any foreign language representation, all statements required by regulation must also appear on the label in foreign language.
- Name and place of Business: Corporate name, Manufactured for XXX, Distributed by XXX, Address, Principal place of business
- Distributor statement: Add manufactured for XXX or Distributed by XXX, if relevant (see above)
- Cosmetic Warning Statements: The FD&C Act and related regulation specify warning and caution statements related to specific products. Cosmetics that may be hazardous to consumers must be bear appropriate label warnings. E.g.: Flammable cosmetics such as aerosols.
- Ingredients declaration: Ingredients must appear clearly and intelligibly, so that an ordinary person under normal conditions of purchase can understand. You must use an appropriate information panel and make sure that the font size is not less than 1/6 inches in height. The IP must be clear for the consumer without obscuring design, vignettes, or crowding. If you do not have sufficient space for such declaration, you may use a tag, tape or card firmly affixed on the package.
- Ingredients name: You may use INCI name or in absence, you should use the name given by the United States Pharmacopeia, National Formulary, Food chemical Codex, USAN, and the USP dictionary of drugs names
- Ingredients listing: They must be listed in descending order of predominance. Exemptions are made for active drug ingredients, ingredients with less than 1% concentration and color additives which can appear in disorder. Fragrance and flavor may be declared in descending order of predominance as “fragrance” and “flavor”.
- Material facts: You should reveal material facts (e.g.: directions for safe use), or your product will be considered as misbranded or adulterated.
Please check the full labeling guide on the FDA website for more detailed information.
Labeling and Trade secret
What is a trade secret?
"A trade secret may consist of any formula, pattern, device or compilation of information which is used in one's business and which gives one an opportunity to obtain an advantage over competitors who do not know or use it."
Cosmetics marketed on a retail basis to consumers must have an ingredient list as mentioned by The Fair Packaging and Labeling Act (FPLA). But under the law, this list cannot be used to make a company disclose “trade secrets”.
Title 21, Code of Federal Regulations (CFR), Part 701.3 (a) give an example:« Fragrance and flavor ingredients do not need to be listed individually on cosmetic labels, because they are the ingredients most likely to be “trade secrets.” Instead, they may be listed simply as “fragrance” or “flavor”.
If you want proceed to a « trade secrets » you have to keep in mind that the FDA only granted once this status.
How to obtain “trade secrets” status?
You’ll have to submit your formation or raw material composition statement to FDA’s VCRP using the “FDA2512” form named Cosmetic product ingredient statement.
You will need to disclose to the FDA the information they need to evaluate the factual and legal grounds justifying trade secrecy for the ingredient in question. You’ll need to address:
- The extent to which the ingredient is known, both outside and inside your company, and what you have done to protect that information, as well as how easily it could be identified or duplicated
- The value of the identity of the ingredient to you or to your competitors, if they became aware of it, as well as the effort and expense involved in developing the ingredient
You will receive at least an interim response from the FDA within 180 days.
European labeling requirements
The EU cosmetic regulation defines the rules for a compliant cosmetic label.
Here is the mandatory information that must be printed in “indelible, easily legible and visible lettering”:
- Name and address of the Responsible Person: If you don’t know the role of the Responsible Person (RP) If you’re outside the EU, it is mandatory to designate an RP in order to market your product. If you’re a cosmetic brand based in the EU, you will be acting as the RP by default, unless you designate someone else.
- Country of origin: Add the mention: “Made in XXXXX” unless the product is made in Europe in which case such mention is not mandatory. Note: the “Made in” expression does not need any translation.
- Nominal content: The nominal content must appear in grams (g) or milliliter (ml) and in first position. You can add additional measurement units if you wish, e.g. oz.
- Date of minimum durability (DOMD) & Period after opening (PAO): If the durability is inferior or equal to 30 months, you need to indicate the “hour glass” symbol and print date (MMYYYY or MMYY or DDMMYYYY or DDMMYY). If the durability is superior to 30 months then you must indicate the PAO. You’ll need to print the “open jar” symbol with the number of months (M) or year (Y) inside or next to the open jar.
- Particular precautions of use and warnings: Depending on the type of cosmetic product, some particular precautions of use and warnings might be useful to consumers or even mandatory in certain cases.
- Batch number: is mandatory, although no particular format is required.
- Product function: The function of the product must be clearly indicated, e.g.: hand moisturizer as to prevent any misuse.
- List of ingredients: In decreasing order of weight, except for ingredients below 1%.
Please note:
- Translation : The European union is composed of 28 countries. There are more than 24 official languages. You must translate the function of the product, the precautions of use and warnings but also the nominal content in the language of the country you export to. Note that Austria, Bulgaria, France, Poland, Portugal and Slovakia, request full translation of the label, i.e. even the marketing content and claims.
- All elements above must be on the labels. When it is impossible to print the mandatory information for practical reasons, i.e. not enough space because your product is too small, then you can use a leaflet for specific information only. A leaflet is an enclosed or attached leaflet, label tape, tag or card. In this case, the information shall be referred to on the primary packaging (PP) and/or secondary packaging (SP) by the « hand-in-book » symbol.
Highlights: key differences between U.S. and EU labeling requirements
Example of a compliant label for the EU market:

Which symbols should I use?

The hour-glass symbol to illustrate the Date of Minimum Durability (DOMD) when equal or below 30 months. The DOMD is defined by the stability test. You must add date near the symbol.
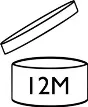
If the DOMD exceeds 30 months, the open-jar symbol will indicate the period after opening "PAO” defined by the combination of the stability test and challenge test.

The hand-in-book symbol will indicate to the consumer that a card, tag or leaflet is enclosed with the product with more regulatory information.
Practical case: Is a sunscreen a cosmetic or a drug?
Answer: it depends which market we consider. In the EU, a sunscreen meets the definition of a cosmetic product as defined by Regulation 1223/2009. Whereas, in the U.S, the FDA considers that a sunscreen is not cosmetic product but an Over-The-Counter ("OTC") drug. As a consumer you can buy it without any prescription but as a manufacturer, you'll have to comply with both regulations (FD&C Act & drug regulation). Also, claims are restricted and labeling will be different (you should inform the consumer of the active ingredients that your product contains).
When consumers see the term “sunscreen” or similar sun protection terminology in the labeling of a product, they expect the product to protect them in some way from the harmful effects of the sun, irrespective of other labeling statements. Consequently, the use of the term “sunscreen” or similar sun protection terminology in a product’s labeling generally causes the product to be subject to regulation as a drug. – FDA Labeling Regulations Applicable to Cosmetics
The FDA provides a guide to better understand this particular case:

The Safe Cosmetics Act versus the EU Cosmetic Regulation
To better understand labeling requirements, let’s start with a bit of context on labeling, packaging and marketing of cosmetic products.
U.S. Regulation
You will usually find two types of laws in the United States: Federal and local regulation. We will focus on Federal laws, and will only mention the most popular local regulations: Proposition 65 in California, California Safety Act, Chemical of High Concern to Children in Washington State, Part I of Chapter 499 of Florida statutes, Wholesale Food & Cosmetic Project in New Jersey.
Cosmetic products are regulated by the Food and Drugs Administration (FDA) through different laws:
- Federal Food Drugs and Cosmetic Act (FD&C) of 1938, as amended
- Fair Packaging and Labeling Act
In 2010, the “Safe Cosmetics Act” gave the FDA power to control chemical substances in cosmetics and the possibility to ban carcinogens and endocrine disruptors. The FD&C Act prohibits the marketing of adulterated or misbranded cosmetics in interstate commerce. A new amendment was scheduled for 2012, and supported by the Republican Leonard Lance.
The Food and Drugs Administration is also competent for color additives. They set up guidelines for manufacturers to assess the safety of their own products:
- Hazards Identification
- Evaluation of the dose-response relationship
- Exposure assessment
- Risk Characterization
To achieve these goals, companies can rely on:
- Available toxicological data tests on similar ingredients and other product formulation
- If necessary, the realization of additional toxicological tests regarding available information and data.
For the moment, there is no regulation related to nanomaterials.
The FD&C Act also includes a reporting program named “Voluntary Cosmetic Registration Program”- (VCRP) for the manufacturers. The VCRP is divided in two parts:
- Registration of companies
- Cosmetic Product Ingredient Statement
If you don’t know how to categorize your product you can read this guide: Is my product a Cosmetic, a drug, or both? (Or is it Soap?)
The EU Cosmetic Regulation
The EU Cosmetic Regulation 1223/2009 came into force in 2013 and concerns 31 European countries (28 countries of the EU + Norway + Iceland + Lichtenstein). The regulation is based on three principles:
- Safety of Raw Materials and Ingredients
- Good manufacturing practices
- Invigilating of cosmetic market
These principles translate into requirements for the cosmetic brand (non-exhaustive list):
- Designate a Responsible Person (RP)
- Prepare a Product Information file (PIF) including a Safety Assessment
- Respect the Good manufacturing practices (GMP) for cosmetics
- Comply with Labeling and Packaging requirements
- Ensure notification via the Cosmetic Products Notification Portal (CPNP)
With this regulation, the European Union sets a higher level of transparency for finished cosmetic products, prevents the placing on the market of hazardous substances, and strengthen safety for consumers.
Labeling requirements: US versus EU
U.S. labeling requirements
The cosmetics distributed in the US must comply with the Cosmetic Labeling Guide published by the FDA under the authority of the FD&C Act. Labeling means all labels and other written, printed or graphic matter on or accompanying a product.
The main requirements are:
Principal Display Panel (PDP)
- Identity statement: The common or usual name, descriptive name, fanciful name, Illustration, Prominence, Placement
- Net quantity: Quantity of the contents, in terms of weight, measure, numerical count
- Warning: If the cosmetic product contains an ingredient for which adequate substantiation of safety has not been obtained, a warning must be placed on the PDP like “Warning - the safety of this product has not been determined”
Please note: The label statements required under the authority of the FD&C Act must appear on the inside as well as any outside container or wrapper.
Information panel (IP):
- Language: All label or labeling statements required by law or regulation must be in English. If the label contains any foreign language representation, all statements required by regulation must also appear on the label in foreign language.
- Name and place of Business: Corporate name, Manufactured for XXX, Distributed by XXX, Address, Principal place of business
- Distributor statement: Add manufactured for XXX or Distributed by XXX, if relevant (see above)
- Cosmetic Warning Statements: The FD&C Act and related regulation specify warning and caution statements related to specific products. Cosmetics that may be hazardous to consumers must be bear appropriate label warnings. E.g.: Flammable cosmetics such as aerosols.
- Ingredients declaration: Ingredients must appear clearly and intelligibly, so that an ordinary person under normal conditions of purchase can understand. You must use an appropriate information panel and make sure that the font size is not less than 1/6 inches in height. The IP must be clear for the consumer without obscuring design, vignettes, or crowding. If you do not have sufficient space for such declaration, you may use a tag, tape or card firmly affixed on the package.
- Ingredients name: You may use INCI name or in absence, you should use the name given by the United States Pharmacopeia, National Formulary, Food chemical Codex, USAN, and the USP dictionary of drugs names
- Ingredients listing: They must be listed in descending order of predominance. Exemptions are made for active drug ingredients, ingredients with less than 1% concentration and color additives which can appear in disorder. Fragrance and flavor may be declared in descending order of predominance as “fragrance” and “flavor”.
- Material facts: You should reveal material facts (e.g.: directions for safe use), or your product will be considered as misbranded or adulterated.
Please check the full labeling guide on the FDA website for more detailed information.
Labeling and Trade secret
What is a trade secret?
"A trade secret may consist of any formula, pattern, device or compilation of information which is used in one's business and which gives one an opportunity to obtain an advantage over competitors who do not know or use it."
Cosmetics marketed on a retail basis to consumers must have an ingredient list as mentioned by The Fair Packaging and Labeling Act (FPLA). But under the law, this list cannot be used to make a company disclose “trade secrets”.
Title 21, Code of Federal Regulations (CFR), Part 701.3 (a) give an example:« Fragrance and flavor ingredients do not need to be listed individually on cosmetic labels, because they are the ingredients most likely to be “trade secrets.” Instead, they may be listed simply as “fragrance” or “flavor”.
If you want proceed to a « trade secrets » you have to keep in mind that the FDA only granted once this status.
How to obtain “trade secrets” status?
You’ll have to submit your formation or raw material composition statement to FDA’s VCRP using the “FDA2512” form named Cosmetic product ingredient statement.
You will need to disclose to the FDA the information they need to evaluate the factual and legal grounds justifying trade secrecy for the ingredient in question. You’ll need to address:
- The extent to which the ingredient is known, both outside and inside your company, and what you have done to protect that information, as well as how easily it could be identified or duplicated
- The value of the identity of the ingredient to you or to your competitors, if they became aware of it, as well as the effort and expense involved in developing the ingredient
You will receive at least an interim response from the FDA within 180 days.
European labeling requirements
The EU cosmetic regulation defines the rules for a compliant cosmetic label.
Here is the mandatory information that must be printed in “indelible, easily legible and visible lettering”:
- Name and address of the Responsible Person: If you don’t know the role of the Responsible Person (RP) If you’re outside the EU, it is mandatory to designate an RP in order to market your product. If you’re a cosmetic brand based in the EU, you will be acting as the RP by default, unless you designate someone else.
- Country of origin: Add the mention: “Made in XXXXX” unless the product is made in Europe in which case such mention is not mandatory. Note: the “Made in” expression does not need any translation.
- Nominal content: The nominal content must appear in grams (g) or milliliter (ml) and in first position. You can add additional measurement units if you wish, e.g. oz.
- Date of minimum durability (DOMD) & Period after opening (PAO): If the durability is inferior or equal to 30 months, you need to indicate the “hour glass” symbol and print date (MMYYYY or MMYY or DDMMYYYY or DDMMYY). If the durability is superior to 30 months then you must indicate the PAO. You’ll need to print the “open jar” symbol with the number of months (M) or year (Y) inside or next to the open jar.
- Particular precautions of use and warnings: Depending on the type of cosmetic product, some particular precautions of use and warnings might be useful to consumers or even mandatory in certain cases.
- Batch number: is mandatory, although no particular format is required.
- Product function: The function of the product must be clearly indicated, e.g.: hand moisturizer as to prevent any misuse.
- List of ingredients: In decreasing order of weight, except for ingredients below 1%.
Please note:
- Translation : The European union is composed of 28 countries. There are more than 24 official languages. You must translate the function of the product, the precautions of use and warnings but also the nominal content in the language of the country you export to. Note that Austria, Bulgaria, France, Poland, Portugal and Slovakia, request full translation of the label, i.e. even the marketing content and claims.
- All elements above must be on the labels. When it is impossible to print the mandatory information for practical reasons, i.e. not enough space because your product is too small, then you can use a leaflet for specific information only. A leaflet is an enclosed or attached leaflet, label tape, tag or card. In this case, the information shall be referred to on the primary packaging (PP) and/or secondary packaging (SP) by the « hand-in-book » symbol.
Highlights: key differences between U.S. and EU labeling requirements
Example of a compliant label for the EU market:

Which symbols should I use?

The hour-glass symbol to illustrate the Date of Minimum Durability (DOMD) when equal or below 30 months. The DOMD is defined by the stability test. You must add date near the symbol.

If the DOMD exceeds 30 months, the open-jar symbol will indicate the period after opening "PAO” defined by the combination of the stability test and challenge test.

The hand-in-book symbol will indicate to the consumer that a card, tag or leaflet is enclosed with the product with more regulatory information.
Practical case: Is a sunscreen a cosmetic or a drug?
Answer: it depends which market we consider. In the EU, a sunscreen meets the definition of a cosmetic product as defined by Regulation 1223/2009. Whereas, in the U.S, the FDA considers that a sunscreen is not cosmetic product but an Over-The-Counter ("OTC") drug. As a consumer you can buy it without any prescription but as a manufacturer, you'll have to comply with both regulations (FD&C Act & drug regulation). Also, claims are restricted and labeling will be different (you should inform the consumer of the active ingredients that your product contains).
When consumers see the term “sunscreen” or similar sun protection terminology in the labeling of a product, they expect the product to protect them in some way from the harmful effects of the sun, irrespective of other labeling statements. Consequently, the use of the term “sunscreen” or similar sun protection terminology in a product’s labeling generally causes the product to be subject to regulation as a drug. – FDA Labeling Regulations Applicable to Cosmetics
The FDA provides a guide to better understand this particular case:








