
Definition
CPNP stands for Cosmetic Products Notification Portal and is the online notification system made mandatory by the EU Cosmetics Regulation No 1223/2009. The CPNP notification was made mandatory on July 11th, 2013. Any cosmetic product must be systematically notified on this portal prior to being placed on the EU market.
Only one notification is necessary to access the EU market and its 31 countries. The European Economic Area is made of the 28 EU Member States + Norway + Iceland + Lichtenstein.
Who can notify a cosmetic product on the CPNP?
The notification of a cosmetic product is set up by the Cosmetics Regulation No 1223/2009 under article 13. The notification is sent electronically to the European Commission prior to placing the cosmetic product on the EU market.
The European Cosmetics Regulation No 1223/2009 requires that Responsible Persons and, under certain circumstances, distributors of cosmetic products submit some information about the products they place or make available on the European market through the CPNP.
NB:The notification is made on behalf of the Responsible Person (RP). The RP is a legal or natural person established within the European Community.
Which information do I need to provide?
The following information is required:
- Category of cosmetic product and its name(s)
- Name and address of the Responsible Person (RP)
- Country of origin in the case of import
- Member State in which the cosmetic product is first placed on the EU market
- Contact details of a physical person to contact in case of necessity
- Presence of substances in the form of nanomaterials
- Identification of the nano-sized substance
- Reasonably foreseeable exposure conditions
- Name and CAS number (Chemical Abstracts Services) or EC number of category 1A or 1B CMR substances (Annex to CLP Regulation (EC) No 1272/2008 on classification, labeling and packaging of substances and mixtures)
- Frame formulation allowing for prompt and appropriate medical treatment in the event of difficulties
Once your cosmetic product is launched on the market, the Responsible Person must provide the European Commission with the original labeling, and, where reasonably legible, a photograph of the corresponding packaging.
Please note: the formula of the cosmetic product can be submitted in three different formats:
- “Frame formulations” notification type: In this case the complete composition of the formulation is not mandatory if the product is considered to have a standardized formula. Only the specific data and the exact concentration of some ingredients at risk must be provided.
- “Exact concentrations” notification type: In this case the composition of the product with the exact concentrations of every ingredient must be provided.
- “Concentration range” notification type: In this case it will be possible to use specific concentration ranges for every ingredient which does not pose any particular threat. The others will be provided with the exact concentrations. Plus, specific data about some substances will also be provided.
Specificity for cosmetic products containing nanomaterials
If the cosmetic product you intend to place on the EU market contains nanomaterials, specific rules apply.
Under article 16, the European Cosmetics Regulation requires a notification six months prior to the launch of the product on the market. You also need to provide additional information:
- Description and specification of the nanomaterial including its chemical name
- The specification of the nanomaterial including size of particles, physical and chemical properties
- Estimate of the quantity of nanomaterial contained in cosmetic products intended to be placed on the market per year
- The toxicological profile of the nanomaterial
- The Safety data of the nanomaterial relating to the category of the cosmetic product
- The reasonably foreseeable exposure conditions
How many notifications do I have to do?
The notification must be done once for each product. There is no need for multiple notifications if the product has different shades.
Particular cases:
If the formula of the product is the same, but there are different packs, e.g. product or brand name, claims, primary packaging type, then you’ll need to perform as many notifications as packs. Also, if the product is imported from several countries, it is necessary to to do as many notifications as countries.
Updating and amending information on the CPNP
It is important to clearly distinguish between the notions of updating and amending a CPNP notification. This distinction is particularly crucial when it comes to the formulation of cosmetic products.
An “update” of the formula means a modification of information which was correct until then. The original data is linked to the old formulation of the product whereas the updated data file is associated to the new formula. Poison control centers will have the possibility to assess both formulas if they cannot identify which one is implicated in an exposure case.
Please note: If all or part of the information provided on the CPNP portal changes, the person who made this notification (Distributor or RP) must provide an update without delay.
An “amendment” of the formula means a modification of wrong or incomplete product information without changing the (real) formulation. Poison control centers will only evaluate the amended data file in that case.
Who can access the information?
Under articles 25, 26 and 27 of the Regulation EC No 1223/2009, the European Commission makes this information available to the competent authorities without delay in order to:
- Monitor the market
- Analyze the market
- Assess and inform consumers
Some of this information is also made available to poison control centers and those alike, when they have been established by the EU Members. This information can only be used by these organizations for medical purposes.
The commercial information, said to be confidential, is only accessible to the company who does the notification. A Responsible Person (RP) or a Distributor notifying via the CPNP will only be able to access the information relating to the products he has entered in the system himself.
In short, the CPNP portal is accessible to:
- Competent Authorities: when checking the data for medical or enforcement purposes
- European Poison Control Centers: when checking the data for medical purposes only
- Cosmetic product Responsible Persons: when notifying their products
- Distributors of cosmetic products: when notifying their products
How do I do my CPNP notification?
The European Commission implemented an online tutorial to assist the concerned actors with their CPNP notification.
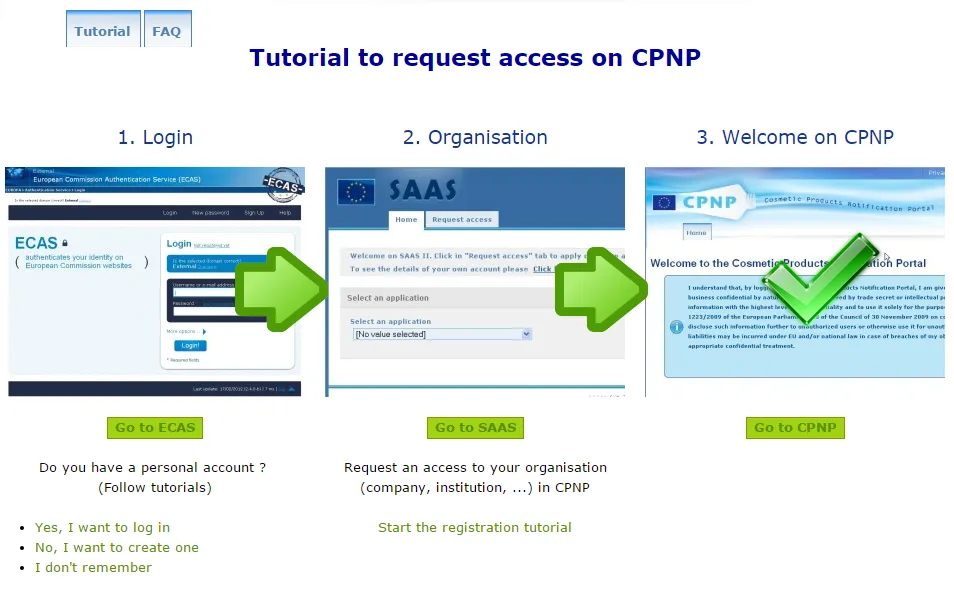
1 – Create an ECAS account
The principle is simple: you must first have an ECAS account (European Commission Authentication Service), which authenticates the users of the European Commission’s offered services.
2 – Access the application to do your CPNP notification
Your ECAS account enables you to access the SAAS application, to make authorization requests for online notifications. Once you’re on this application, you just have to choose to do the CPNP notification and request access.
3 – Do your notification
Then you must fill the information described in Article 13 of the Cosmetics Regulations No 1223/2009 about your company, whether it already exists in the system or not.
NB:The notification is free of charge.
Definition
CPNP stands for Cosmetic Products Notification Portal and is the online notification system made mandatory by the EU Cosmetics Regulation No 1223/2009. The CPNP notification was made mandatory on July 11th, 2013. Any cosmetic product must be systematically notified on this portal prior to being placed on the EU market.
Only one notification is necessary to access the EU market and its 31 countries. The European Economic Area is made of the 28 EU Member States + Norway + Iceland + Lichtenstein.
Who can notify a cosmetic product on the CPNP?
The notification of a cosmetic product is set up by the Cosmetics Regulation No 1223/2009 under article 13. The notification is sent electronically to the European Commission prior to placing the cosmetic product on the EU market.
The European Cosmetics Regulation No 1223/2009 requires that Responsible Persons and, under certain circumstances, distributors of cosmetic products submit some information about the products they place or make available on the European market through the CPNP.
NB:The notification is made on behalf of the Responsible Person (RP). The RP is a legal or natural person established within the European Community.
Which information do I need to provide?
The following information is required:
- Category of cosmetic product and its name(s)
- Name and address of the Responsible Person (RP)
- Country of origin in the case of import
- Member State in which the cosmetic product is first placed on the EU market
- Contact details of a physical person to contact in case of necessity
- Presence of substances in the form of nanomaterials
- Identification of the nano-sized substance
- Reasonably foreseeable exposure conditions
- Name and CAS number (Chemical Abstracts Services) or EC number of category 1A or 1B CMR substances (Annex to CLP Regulation (EC) No 1272/2008 on classification, labeling and packaging of substances and mixtures)
- Frame formulation allowing for prompt and appropriate medical treatment in the event of difficulties
Once your cosmetic product is launched on the market, the Responsible Person must provide the European Commission with the original labeling, and, where reasonably legible, a photograph of the corresponding packaging.
Please note: the formula of the cosmetic product can be submitted in three different formats:
- “Frame formulations” notification type: In this case the complete composition of the formulation is not mandatory if the product is considered to have a standardized formula. Only the specific data and the exact concentration of some ingredients at risk must be provided.
- “Exact concentrations” notification type: In this case the composition of the product with the exact concentrations of every ingredient must be provided.
- “Concentration range” notification type: In this case it will be possible to use specific concentration ranges for every ingredient which does not pose any particular threat. The others will be provided with the exact concentrations. Plus, specific data about some substances will also be provided.
Specificity for cosmetic products containing nanomaterials
If the cosmetic product you intend to place on the EU market contains nanomaterials, specific rules apply.
Under article 16, the European Cosmetics Regulation requires a notification six months prior to the launch of the product on the market. You also need to provide additional information:
- Description and specification of the nanomaterial including its chemical name
- The specification of the nanomaterial including size of particles, physical and chemical properties
- Estimate of the quantity of nanomaterial contained in cosmetic products intended to be placed on the market per year
- The toxicological profile of the nanomaterial
- The Safety data of the nanomaterial relating to the category of the cosmetic product
- The reasonably foreseeable exposure conditions
How many notifications do I have to do?
The notification must be done once for each product. There is no need for multiple notifications if the product has different shades.
Particular cases:
If the formula of the product is the same, but there are different packs, e.g. product or brand name, claims, primary packaging type, then you’ll need to perform as many notifications as packs. Also, if the product is imported from several countries, it is necessary to to do as many notifications as countries.
Updating and amending information on the CPNP
It is important to clearly distinguish between the notions of updating and amending a CPNP notification. This distinction is particularly crucial when it comes to the formulation of cosmetic products.
An “update” of the formula means a modification of information which was correct until then. The original data is linked to the old formulation of the product whereas the updated data file is associated to the new formula. Poison control centers will have the possibility to assess both formulas if they cannot identify which one is implicated in an exposure case.
Please note: If all or part of the information provided on the CPNP portal changes, the person who made this notification (Distributor or RP) must provide an update without delay.
An “amendment” of the formula means a modification of wrong or incomplete product information without changing the (real) formulation. Poison control centers will only evaluate the amended data file in that case.
Who can access the information?
Under articles 25, 26 and 27 of the Regulation EC No 1223/2009, the European Commission makes this information available to the competent authorities without delay in order to:
- Monitor the market
- Analyze the market
- Assess and inform consumers
Some of this information is also made available to poison control centers and those alike, when they have been established by the EU Members. This information can only be used by these organizations for medical purposes.
The commercial information, said to be confidential, is only accessible to the company who does the notification. A Responsible Person (RP) or a Distributor notifying via the CPNP will only be able to access the information relating to the products he has entered in the system himself.
In short, the CPNP portal is accessible to:
- Competent Authorities: when checking the data for medical or enforcement purposes
- European Poison Control Centers: when checking the data for medical purposes only
- Cosmetic product Responsible Persons: when notifying their products
- Distributors of cosmetic products: when notifying their products
How do I do my CPNP notification?
The European Commission implemented an online tutorial to assist the concerned actors with their CPNP notification.

1 – Create an ECAS account
The principle is simple: you must first have an ECAS account (European Commission Authentication Service), which authenticates the users of the European Commission’s offered services.
2 – Access the application to do your CPNP notification
Your ECAS account enables you to access the SAAS application, to make authorization requests for online notifications. Once you’re on this application, you just have to choose to do the CPNP notification and request access.
3 – Do your notification
Then you must fill the information described in Article 13 of the Cosmetics Regulations No 1223/2009 about your company, whether it already exists in the system or not.
NB:The notification is free of charge.




.png)


