.png)
Since September 1, 2013, the new Biocidal Products Regulation (BPR) EU No. 528/2012 replaces the Biocidal Products Directive (BPD) (98/8/EC).
Biocidal products are divided into four major groups, comprising 22 product types (TP):
- Disinfectants : TP 1 to 5
- Curators: TP 6 to 13
- Pest control : TP 14 to 20
- Other biocidal products: TP 21 to 22
The establishment of the BPR also allowed a revision of the procedures for the approval of active substances at EU level and for the authorization of biocidal products in the Member States. From now on, only products containing active substances under evaluation or approved by ECHA for a specific TP can be placed on the European market.
In this article, we will focus on 2 major points:
- European Commission extends BPR approval for two biocidal substances
- Ten substances lose BPR approval, European Commission confirms
European Commission extends BPR approval for two biocidal substances
The European Commission has announced a decision to extend the expiry date of the approvals of the neonicotinoid insecticide imidacloprid and the wood preservative 4,5-dichloro-2-octyl-2H-isothiazol-3-one (DCOIT).
In both cases, the EU officials expect the approval to expire before a decision is taken on whether to renew the substance.
The validity of authorizations under the Biocidal Products Regulation (BPR) will be extended from 30 June 2023 to 31 December 2025.
The imidacloprid enforcement decision will take effect on the 23rd March, and DCOIT the 26th March.
Ten substances lose BPR approval, European Commission confirms
The European Commission has listed 10 active substances that are no longer approved for certain uses under the Biocidal Products Regulation (BPR).
The first substance, 2,2-dibromo-2-cyanoacetamide (DBNPA), is used for disinfecting food and feed areas (product-type four). Due to its endocrine disrupting properties, DBNPA will only be approved if authorities identify a need for it, such as a lack of effective alternatives.
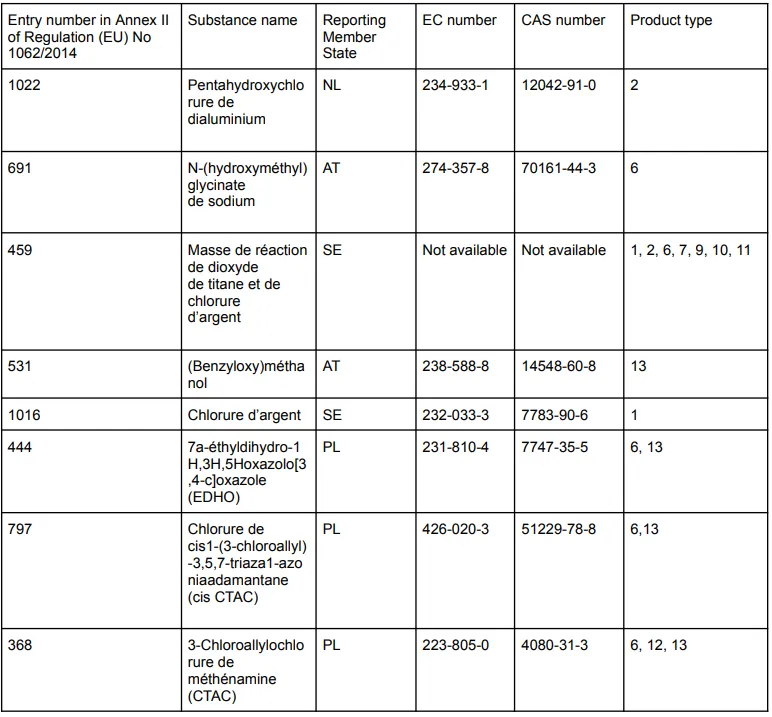
Non-approved active substance/product type combinations
Relation with EcoMundo
At each step we guarantee you a personalized support respecting your deadlines and your confidentiality
1. Strategic diagnosis and cost evaluation
EcoMundo makes an inventory of your obligations and proposes the best strategies adapted to your products and markets, in order to minimize your costs.
2. Risk assessment of the biocidal product
- Consideration of reports from the competent authority on active substances
- Identification of substances of concern
- Exposure modeling
- Risk assessment for human health and the environment
- Follow-up of the approval with the Competent Authorities
3. Preparation of the dossier and submission
EcoMundo supports you from the creation to the submission of your dossier:
- Determine the costs applied to the size of your company
- Realize the " Data gap " of the tox and ecotox data to provide (read-accross, QSAR...)
- Assist in writing the scientific argument
- Assist in the collection of relevant data: analytical, efficacy, toxicology, eco-toxicology, residue analysis etc.
- Fill in the dossier in IUCLID format and generate the dossier
- Submit the dossier via R4BP
4. Intermediary role
EcoMundo defends your interests with :
- ECHA and BPC (Biocidal Product Committee)
- Task forces and consortia
- Data owners to negotiate the costs of LoAs
- Member State evaluation to validate the submission authorization and manage exchanges in case of additional information request.
Since September 1, 2013, the new Biocidal Products Regulation (BPR) EU No. 528/2012 replaces the Biocidal Products Directive (BPD) (98/8/EC).
Biocidal products are divided into four major groups, comprising 22 product types (TP):
- Disinfectants : TP 1 to 5
- Curators: TP 6 to 13
- Pest control : TP 14 to 20
- Other biocidal products: TP 21 to 22
The establishment of the BPR also allowed a revision of the procedures for the approval of active substances at EU level and for the authorization of biocidal products in the Member States. From now on, only products containing active substances under evaluation or approved by ECHA for a specific TP can be placed on the European market.
In this article, we will focus on 2 major points:
- European Commission extends BPR approval for two biocidal substances
- Ten substances lose BPR approval, European Commission confirms
European Commission extends BPR approval for two biocidal substances
The European Commission has announced a decision to extend the expiry date of the approvals of the neonicotinoid insecticide imidacloprid and the wood preservative 4,5-dichloro-2-octyl-2H-isothiazol-3-one (DCOIT).
In both cases, the EU officials expect the approval to expire before a decision is taken on whether to renew the substance.
The validity of authorizations under the Biocidal Products Regulation (BPR) will be extended from 30 June 2023 to 31 December 2025.
The imidacloprid enforcement decision will take effect on the 23rd March, and DCOIT the 26th March.
Ten substances lose BPR approval, European Commission confirms
The European Commission has listed 10 active substances that are no longer approved for certain uses under the Biocidal Products Regulation (BPR).
The first substance, 2,2-dibromo-2-cyanoacetamide (DBNPA), is used for disinfecting food and feed areas (product-type four). Due to its endocrine disrupting properties, DBNPA will only be approved if authorities identify a need for it, such as a lack of effective alternatives.
Non-approved active substance/product type combinations

Relation with EcoMundo
At each step we guarantee you a personalized support respecting your deadlines and your confidentiality
1. Strategic diagnosis and cost evaluation
EcoMundo makes an inventory of your obligations and proposes the best strategies adapted to your products and markets, in order to minimize your costs.
2. Risk assessment of the biocidal product
- Consideration of reports from the competent authority on active substances
- Identification of substances of concern
- Exposure modeling
- Risk assessment for human health and the environment
- Follow-up of the approval with the Competent Authorities
3. Preparation of the dossier and submission
EcoMundo supports you from the creation to the submission of your dossier:
- Determine the costs applied to the size of your company
- Realize the " Data gap " of the tox and ecotox data to provide (read-accross, QSAR...)
- Assist in writing the scientific argument
- Assist in the collection of relevant data: analytical, efficacy, toxicology, eco-toxicology, residue analysis etc.
- Fill in the dossier in IUCLID format and generate the dossier
- Submit the dossier via R4BP
4. Intermediary role
EcoMundo defends your interests with :
- ECHA and BPC (Biocidal Product Committee)
- Task forces and consortia
- Data owners to negotiate the costs of LoAs
- Member State evaluation to validate the submission authorization and manage exchanges in case of additional information request.







