
The EU Cosmetic Regulation
Enforced in 2013, the EU Cosmetic Regulation 1223/2009 concerns 31 European countries (28 countries of the EU + Norway + Iceland + Lichtenstein). The Cosmetic Regulation is the main regulatory framework for finished cosmetic products when placed on the EU market. It ensure that cosmetic placed through the EU market are safe for the consumer
Definitions
Cosmetic product: “Means any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odours".

Labeling: Mandatory requirements
Article 19 of the EU cosmetic regulation defines the rules for a compliant cosmetic label.
Here is the mandatory information that must be printed in “indelible, easily legible and visible lettering”:
- Name and address of the Responsible Person: If you don’t know the role of the Responsible Person (RP. If you’re outside the EU, it is mandatory to designate a RP based in Europe in order to market your product. If you’re a cosmetic brand based in the EU, you will be acting as the RP by default, unless you designate someone else.
- Country of origin: Add the words: “Made in XXXXX” unless the product is made in Europe in which case this is not mandatory. Note: the “Made in” expression does not need any translation.
- Nominal content: The nominal content must appear in grams (g) or milliliter (ml) and in first position. You can add additional measurement units if you wish, e.g. oz.
- Date of minimum durability (DOMD) & Period after opening (PAO): If the DOMD (defined by the stability test) is inferior or equal to 30 months, you need to indicate it using the “hour glass” symbol and print date (MMYYYY or MMYY or DDMMYYYY or DDMMYY). If the DOMD is superior to 30 months then you must indicate the PAO, defined by the challenge test. You’ll need to print the “open jar” symbol with the number of months (M) or year (Y) inside or next to the open jar.
- Particular precautions of use and warnings: Depending on the type of cosmetic product, some particular precautions of use and warnings might be useful to consumers or even mandatory in certain cases, e.g. avoid contact with the eyes.
- Batch number: is mandatory, and no particular format is required.
- Product function: The function of the product must be clearly indicated, e.g. hand moisturizer so as to prevent any misuse.If you have any doubt about the primary function of your product, please consult our article on how to classify borderline cosmetics.
- List of ingredients: In decreasing order of weight, except for ingredients below 1%.
Please note:
- Translation: The EU Cosmetic Regulation applies to 31 EU countries representing more than 24 different official languages. You must translate the function of the product, the precautions of use and warnings but also the nominal content in the language of the country you export to. Note that Austria, Bulgaria, France, Poland, Portugal and Slovakia, request full translation of the label, i.e. even the marketing content and claims.
- All elements above must be on the labels. When it is impossible to print the mandatory information for practical reasons, i.e. not enough space because your product is too small, then you can use a leaflet for specific information only. A leaflet is an enclosed or attached leaflet, label tape, tag or card. In this case, the information shall be referred to on the primary packaging (PP) and/or secondary packaging (SP) by the « hand-in-book » symbol.
Symbols requirements

The hour-glass symbol to illustrate the Date of Minimum Durability (DOMD) when equal or below 30 months. The DOMD is defined by the stability test. You must add the date near the symbol
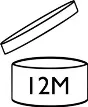
If the DOMD exceeds 30 months, the open-jar symbol will indicate the period after opening "PAO” defined by the combination of the stability test and challenge test.

The hand-in-book symbol will indicate to the consumer that a card, tag or leaflet is enclosed with the product with more regulatory information.
Example of a compliant label
Claims
Cosmetic product claims are mentioned in article 20 of Regulation 1223/2009 but also in a specific regulation dedicated to claims EC No. 655/2013. This regulation aims to ensure that the information conveyed to the end users through claims is useful, understandable and reliable. It must enable them to make informed decisions and to choose the product that best suits their needs and expectations.
Cosmetic claim are usually used to market the final product. Claims will appear on the label but also on ads, magazines, etc. They:
- Describe the effects of a product
- Help consumers/users choose a product
- Make the product seem more appealing than the competition
6 common criteria
- Legal compliance
- Truthfulness
- Evidential support
- Honesty
- Fairness
- Informed decision-making
EcoMundo's services
- Regulation 1223/2009 services
- EU cosmetic compliance
- Responsible Person for Europe
- Formula review
- Claim review
- Cosmetic labeling services
- PIF creation
- Safety Assessment
- CPNP notification services
- Cosmetic certification for Europe
The EU Cosmetic Regulation
Enforced in 2013, the EU Cosmetic Regulation 1223/2009 concerns 31 European countries (28 countries of the EU + Norway + Iceland + Lichtenstein). The Cosmetic Regulation is the main regulatory framework for finished cosmetic products when placed on the EU market. It ensure that cosmetic placed through the EU market are safe for the consumer
Definitions
Cosmetic product: “Means any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odours".

Labeling: Mandatory requirements
Article 19 of the EU cosmetic regulation defines the rules for a compliant cosmetic label.
Here is the mandatory information that must be printed in “indelible, easily legible and visible lettering”:
- Name and address of the Responsible Person: If you don’t know the role of the Responsible Person (RP. If you’re outside the EU, it is mandatory to designate a RP based in Europe in order to market your product. If you’re a cosmetic brand based in the EU, you will be acting as the RP by default, unless you designate someone else.
- Country of origin: Add the words: “Made in XXXXX” unless the product is made in Europe in which case this is not mandatory. Note: the “Made in” expression does not need any translation.
- Nominal content: The nominal content must appear in grams (g) or milliliter (ml) and in first position. You can add additional measurement units if you wish, e.g. oz.
- Date of minimum durability (DOMD) & Period after opening (PAO): If the DOMD (defined by the stability test) is inferior or equal to 30 months, you need to indicate it using the “hour glass” symbol and print date (MMYYYY or MMYY or DDMMYYYY or DDMMYY). If the DOMD is superior to 30 months then you must indicate the PAO, defined by the challenge test. You’ll need to print the “open jar” symbol with the number of months (M) or year (Y) inside or next to the open jar.
- Particular precautions of use and warnings: Depending on the type of cosmetic product, some particular precautions of use and warnings might be useful to consumers or even mandatory in certain cases, e.g. avoid contact with the eyes.
- Batch number: is mandatory, and no particular format is required.
- Product function: The function of the product must be clearly indicated, e.g. hand moisturizer so as to prevent any misuse.If you have any doubt about the primary function of your product, please consult our article on how to classify borderline cosmetics.
- List of ingredients: In decreasing order of weight, except for ingredients below 1%.
Please note:
- Translation: The EU Cosmetic Regulation applies to 31 EU countries representing more than 24 different official languages. You must translate the function of the product, the precautions of use and warnings but also the nominal content in the language of the country you export to. Note that Austria, Bulgaria, France, Poland, Portugal and Slovakia, request full translation of the label, i.e. even the marketing content and claims.
- All elements above must be on the labels. When it is impossible to print the mandatory information for practical reasons, i.e. not enough space because your product is too small, then you can use a leaflet for specific information only. A leaflet is an enclosed or attached leaflet, label tape, tag or card. In this case, the information shall be referred to on the primary packaging (PP) and/or secondary packaging (SP) by the « hand-in-book » symbol.
Symbols requirements

The hour-glass symbol to illustrate the Date of Minimum Durability (DOMD) when equal or below 30 months. The DOMD is defined by the stability test. You must add the date near the symbol

If the DOMD exceeds 30 months, the open-jar symbol will indicate the period after opening "PAO” defined by the combination of the stability test and challenge test.

The hand-in-book symbol will indicate to the consumer that a card, tag or leaflet is enclosed with the product with more regulatory information.
Example of a compliant label
Claims
Cosmetic product claims are mentioned in article 20 of Regulation 1223/2009 but also in a specific regulation dedicated to claims EC No. 655/2013. This regulation aims to ensure that the information conveyed to the end users through claims is useful, understandable and reliable. It must enable them to make informed decisions and to choose the product that best suits their needs and expectations.
Cosmetic claim are usually used to market the final product. Claims will appear on the label but also on ads, magazines, etc. They:
- Describe the effects of a product
- Help consumers/users choose a product
- Make the product seem more appealing than the competition
6 common criteria
- Legal compliance
- Truthfulness
- Evidential support
- Honesty
- Fairness
- Informed decision-making
EcoMundo's services
- Regulation 1223/2009 services
- EU cosmetic compliance
- Responsible Person for Europe
- Formula review
- Claim review
- Cosmetic labeling services
- PIF creation
- Safety Assessment
- CPNP notification services
- Cosmetic certification for Europe




.png)


