
Microplastics: Concerns and Regulatory Actions in the EU
Problematic over Microplastics
Microplastics are minuscule solid plastic particles that can be intentionally added to products or form unintentionally as larger plastic materials degrade. In the context of cosmetics, microplastics are often used as abrasive agents, exfoliating beads, film former or for controlling product thickness and appearance.
Microplastics pose significant challenges to ecosystems and human health. Their persistence and accumulation in the environment contribute to permanent pollution. These particles have been found in marine, freshwater, and terrestrial ecosystems, as well as in food and drinking water. Exposure to microplastics has been linked to adverse effects on living organisms, raising concerns about their potential toxicity.
The EU's Response and Need for Regulation
Prompted by growing concerns over the environmental and health impacts of microplastics, several EU Member States have already taken action by implementing or proposing national bans on microplastics in cosmetics, particularly microbeads in rinse-off products. To establish a harmonized approach, the EU Commission requested the European Chemicals Agency (ECHA) to assess the scientific evidence and propose regulatory measures for intentional microplastics.
Proposed Restriction and Exemptions
The Commission's approved proposal entails a restriction on the placement of synthetic polymer microparticles (SPM) in cosmetics on the market. If the concentration of these microparticles used for sought-after characteristics exceeds 0.01% by weight, they will be prohibited. The definition of SPM includes specific criteria related to particle size, weight percentage, and polymer composition. Some polymers, such as those without carbon atoms or with high water solubility, degradability or for specific uses (SPM for use at industrial sites, medicinal products, EU fertilizing products, in vitro diagnostic devices, food), are excluded from this definition.
Furthermore, the regulation highlights that certain SPM can be excluded from the concentration limit for the following cases:
- SPM which are contained by technical means so that releases to the environment are prevented when used in accordance with the instructions for use during the intended end use.
- SPM the physical properties of which are permanently modified during intended end use.
- SPM which are permanently incorporated into a solid matrix during intended end use.
__ Good to know:
There are concerns on the real application of this regulation toward microplastics in products. Indeed, the definition of microplastics might not encompass the majority of currently used microplastics. In fact, only 4% of ingredients within this category will face restrictions, leaving 96% of microplastic pollution unaddressed (based on a recent study of the Plastic Soup foundation). Yet, based on the same study, 87% of all cosmetic products contain microplastic.
Implementation Timeline
The EU Commission has established a phased timeline for implementing the intentional microplastic ban per category of cosmetic products, depending on the impact of the environment, the necessity of use in products and the lack of alternatives for industries.
4 years: Transitional Periods for 'Rinse-off' Products
Reminder: Ban of microbeads in rinse-off Products
Microbeads ban in rinse-off cosmetic products, which came into effect on July 1, 2018, represents a proactive step to safeguard the environment. It strictly prohibits the manufacture and sale of rinse-off cosmetic products containing intentionally added microbeads.
Excluding the already banned microbeads, a transitional period of 4 years has been established. During this period, manufacturers are expected to reformulate their products to remove synthetic polymer microparticles effectively.
5 Years: Transitional Periods for Homecare Products (Detergents, Waxes, Polishes, and Air Care Products)
For products like detergents, waxes, polishes, and air care products, a transitional period of 5 years was deemed appropriate. This period allowed manufacturers sufficient time to reformulate their products and seek alternatives to microplastics.
__ Homecare Products containing encapsulated fragrances with microplastics are subjected to the corresponding transition-period of encapsulated fragrance (6 years).
6 years: Transitional Periods for 'Leave-on' Products and Encapsulated Fragrance
Leave-on cosmetic products are subject to a longer transitional period of 6 years. This extended time frame provides ample opportunity for the industry to transition away from synthetic polymer microparticles, aligning with sustainability goals and reducing their environmental impact.
Concerning the use of synthetic microplastic in encapsulated fragrances, the Commission deemed a 6-year transitional period the most appropriate. This timeframe provided the industry with ample time to reformulate products where no alternatives currently exist.
__ Encapsulated fragrances are commonly incorporated into various products, including laundry detergents, fabric softeners, and some cosmetic products, for a specific purpose: to provide a prolonged and enduring fragrance experience. These tiny capsules contain fragrance oils or compounds, and they are designed to release their scent gradually over time.
12-Years: Transitional Periods for Make-up, lip and Nail Products
The reformulation costs for make-up, lip, and nail 'leave-on' cosmetic products are higher than other 'leave-on' products. Given their lower contribution to overall emissions, the Commission justified a transitional period of 12 years for banning such products. This extended period aimed to facilitate the development of suitable alternatives and mitigate industry costs. Additionally, to encourage early substitution of synthetic polymer microparticles in these products, any remaining products after a specified date (8 years from the entry into force of the regulation) were required to bear a label informing consumers of their content.
The most common example of intentional microplastic in makeup and nail products: glitters !
Indeed, glitters are predominantly made of plastic, often a combination of aluminum and PET. As of now there exist no sustainable alternatives for glitters.
__ Make-up and Nail Products containing encapsulated fragrances with microplastics are subjected to the corresponding transition-period of encapsulated fragrance (6 years).
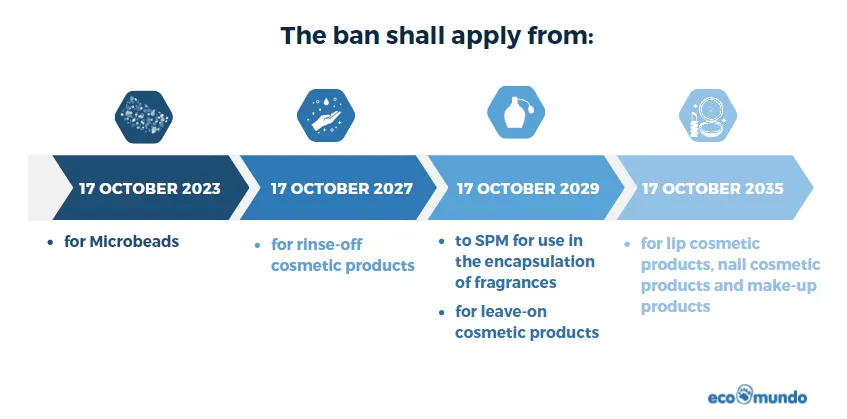
This timeline allows for a gradual transition, giving the industry time to adapt and explore alternative solutions.
Instructions for Use and Disposal
The instructions for use and disposal serve as a practical and environmentally conscious bridge between the transition periods and the long-term goal of reducing the environmental impact of SPM. They ensure that products containing microplastics are used in a way that minimizes their release into the environment. A 24th-month grace period after publication of the regulations was accorded to companies for the reporting requirements.
Requirement for Instructions
Suppliers of products containing SPM (e.g., cosmetics, detergents) must provide clear and comprehensive instructions for the proper use and disposal of these products.
The instructions should cover important aspects, such as:
- Safe and responsible product use.
- How to avoid or minimize environmental releases.
- Proper disposal methods that prevent environmental contamination (environmental logo).
- Any specific precautions or guidelines unique to the product.
Those instructions for use and disposal should be provided in the official languages of the EU Member States where the product is placed on the market, unless specific arrangements are made by the Member States. And these labeling requirements only apply to SPM that loses its microplastic properties during the intended end use (from 17 October 2025)
__ Good to know
- Fast implementation incentives for makeup and nail products: To encourage the substitution of microplastics in make-up and nail products before the 12 years transition period, any make-up and nail product still containing microplastics should have the label. This product contains microplastics informing consumers of this fact. The label must be translated into the national languages, 8 years after the application of the amendment. (NB: products placed on the market before 17 October 2031 are not required to bear that statement until 17 December 2031.)
- Digital Access: Suppliers may also offer a digital tool that provides access to an electronic version of the instructions. This acknowledges the use of modern technology for disseminating information to consumers.
Reporting Requirements (when exempted for use)
Information Submission
Manufacturers and industrial downstream users of synthetic polymer microparticles in various forms (e.g. pellets, flakes, powders) used as feedstock in plastic manufacturing at industrial sites will have to report and submit information to ECHA (the European Chemical Agency).
- Description of uses: they should provide a description of how SPM were used in the previous calendar year. This information helps track and understand the range of applications.
- Polymer identity: for each use of SPM, generic information on the identity of the polymers used should be included. This helps authorities categorize and monitor different types of microparticles.
- Quantity estimates: manufacturers and users must estimate the quantity of SPM released into the environment during the previous calendar year for each end use. This estimate assists in assessing environmental impact.
Reporting obligation is typically required annually, with deadlines for submission set by regulatory authorities (different depending on your company business).
- Starting from 2026 by 31 May of each year for manufacturers and industrial downstream users of SPM in the form of pellets, flakes, and powders
- Starting from 2027 by 31 May of each year for other manufacturers and industrial downstream of SPM at industrial sites.
- Starting from 2027 by 31 May of each year for suppliers of products containing SPM that can be derogated. (end uses, applicable derogation should be submitted)
__ Good to know
Starting from 2025, the suppliers of SPM of excluded product categories are required to inform the downstream user of the presence of microplastics as well as providing appropriate instructions for use according the reporting requirement stating "The synthetic polymer microparticles supplied is subject to conditions laid down by entry 78 of Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council".
The EU Commission's decision to restrict intentional microplastics in cosmetics marks a crucial step toward mitigating the environmental and health risks associated with these particles. By gradually phasing out the use of synthetic polymer microparticles, the EU aims to minimize their release into the environment and reduce their presence in ecosystems and the food chain. The amendment underscores the EU's commitment to sustainable practices in the cosmetics industry and sets an example for other regions to address the issue of microplastics comprehensively.
Would you like to find out more about cosmetics regulations?
Don't hesitate to contact us via our contact form.
_
Microplastics: Concerns and Regulatory Actions in the EU
Problematic over Microplastics
Microplastics are minuscule solid plastic particles that can be intentionally added to products or form unintentionally as larger plastic materials degrade. In the context of cosmetics, microplastics are often used as abrasive agents, exfoliating beads, film former or for controlling product thickness and appearance.
Microplastics pose significant challenges to ecosystems and human health. Their persistence and accumulation in the environment contribute to permanent pollution. These particles have been found in marine, freshwater, and terrestrial ecosystems, as well as in food and drinking water. Exposure to microplastics has been linked to adverse effects on living organisms, raising concerns about their potential toxicity.
The EU's Response and Need for Regulation
Prompted by growing concerns over the environmental and health impacts of microplastics, several EU Member States have already taken action by implementing or proposing national bans on microplastics in cosmetics, particularly microbeads in rinse-off products. To establish a harmonized approach, the EU Commission requested the European Chemicals Agency (ECHA) to assess the scientific evidence and propose regulatory measures for intentional microplastics.
Proposed Restriction and Exemptions
The Commission's approved proposal entails a restriction on the placement of synthetic polymer microparticles (SPM) in cosmetics on the market. If the concentration of these microparticles used for sought-after characteristics exceeds 0.01% by weight, they will be prohibited. The definition of SPM includes specific criteria related to particle size, weight percentage, and polymer composition. Some polymers, such as those without carbon atoms or with high water solubility, degradability or for specific uses (SPM for use at industrial sites, medicinal products, EU fertilizing products, in vitro diagnostic devices, food), are excluded from this definition.
Furthermore, the regulation highlights that certain SPM can be excluded from the concentration limit for the following cases:
- SPM which are contained by technical means so that releases to the environment are prevented when used in accordance with the instructions for use during the intended end use.
- SPM the physical properties of which are permanently modified during intended end use.
- SPM which are permanently incorporated into a solid matrix during intended end use.
__ Good to know:
There are concerns on the real application of this regulation toward microplastics in products. Indeed, the definition of microplastics might not encompass the majority of currently used microplastics. In fact, only 4% of ingredients within this category will face restrictions, leaving 96% of microplastic pollution unaddressed (based on a recent study of the Plastic Soup foundation). Yet, based on the same study, 87% of all cosmetic products contain microplastic.
Implementation Timeline
The EU Commission has established a phased timeline for implementing the intentional microplastic ban per category of cosmetic products, depending on the impact of the environment, the necessity of use in products and the lack of alternatives for industries.
4 years: Transitional Periods for 'Rinse-off' Products
Reminder: Ban of microbeads in rinse-off Products
Microbeads ban in rinse-off cosmetic products, which came into effect on July 1, 2018, represents a proactive step to safeguard the environment. It strictly prohibits the manufacture and sale of rinse-off cosmetic products containing intentionally added microbeads.
Excluding the already banned microbeads, a transitional period of 4 years has been established. During this period, manufacturers are expected to reformulate their products to remove synthetic polymer microparticles effectively.
5 Years: Transitional Periods for Homecare Products (Detergents, Waxes, Polishes, and Air Care Products)
For products like detergents, waxes, polishes, and air care products, a transitional period of 5 years was deemed appropriate. This period allowed manufacturers sufficient time to reformulate their products and seek alternatives to microplastics.
__ Homecare Products containing encapsulated fragrances with microplastics are subjected to the corresponding transition-period of encapsulated fragrance (6 years).
6 years: Transitional Periods for 'Leave-on' Products and Encapsulated Fragrance
Leave-on cosmetic products are subject to a longer transitional period of 6 years. This extended time frame provides ample opportunity for the industry to transition away from synthetic polymer microparticles, aligning with sustainability goals and reducing their environmental impact.
Concerning the use of synthetic microplastic in encapsulated fragrances, the Commission deemed a 6-year transitional period the most appropriate. This timeframe provided the industry with ample time to reformulate products where no alternatives currently exist.
__ Encapsulated fragrances are commonly incorporated into various products, including laundry detergents, fabric softeners, and some cosmetic products, for a specific purpose: to provide a prolonged and enduring fragrance experience. These tiny capsules contain fragrance oils or compounds, and they are designed to release their scent gradually over time.
12-Years: Transitional Periods for Make-up, lip and Nail Products
The reformulation costs for make-up, lip, and nail 'leave-on' cosmetic products are higher than other 'leave-on' products. Given their lower contribution to overall emissions, the Commission justified a transitional period of 12 years for banning such products. This extended period aimed to facilitate the development of suitable alternatives and mitigate industry costs. Additionally, to encourage early substitution of synthetic polymer microparticles in these products, any remaining products after a specified date (8 years from the entry into force of the regulation) were required to bear a label informing consumers of their content.
The most common example of intentional microplastic in makeup and nail products: glitters !
Indeed, glitters are predominantly made of plastic, often a combination of aluminum and PET. As of now there exist no sustainable alternatives for glitters.
__ Make-up and Nail Products containing encapsulated fragrances with microplastics are subjected to the corresponding transition-period of encapsulated fragrance (6 years).

This timeline allows for a gradual transition, giving the industry time to adapt and explore alternative solutions.
Instructions for Use and Disposal
The instructions for use and disposal serve as a practical and environmentally conscious bridge between the transition periods and the long-term goal of reducing the environmental impact of SPM. They ensure that products containing microplastics are used in a way that minimizes their release into the environment. A 24th-month grace period after publication of the regulations was accorded to companies for the reporting requirements.
Requirement for Instructions
Suppliers of products containing SPM (e.g., cosmetics, detergents) must provide clear and comprehensive instructions for the proper use and disposal of these products.
The instructions should cover important aspects, such as:
- Safe and responsible product use.
- How to avoid or minimize environmental releases.
- Proper disposal methods that prevent environmental contamination (environmental logo).
- Any specific precautions or guidelines unique to the product.
Those instructions for use and disposal should be provided in the official languages of the EU Member States where the product is placed on the market, unless specific arrangements are made by the Member States. And these labeling requirements only apply to SPM that loses its microplastic properties during the intended end use (from 17 October 2025)
__ Good to know
- Fast implementation incentives for makeup and nail products: To encourage the substitution of microplastics in make-up and nail products before the 12 years transition period, any make-up and nail product still containing microplastics should have the label. This product contains microplastics informing consumers of this fact. The label must be translated into the national languages, 8 years after the application of the amendment. (NB: products placed on the market before 17 October 2031 are not required to bear that statement until 17 December 2031.)
- Digital Access: Suppliers may also offer a digital tool that provides access to an electronic version of the instructions. This acknowledges the use of modern technology for disseminating information to consumers.
Reporting Requirements (when exempted for use)
Information Submission
Manufacturers and industrial downstream users of synthetic polymer microparticles in various forms (e.g. pellets, flakes, powders) used as feedstock in plastic manufacturing at industrial sites will have to report and submit information to ECHA (the European Chemical Agency).
- Description of uses: they should provide a description of how SPM were used in the previous calendar year. This information helps track and understand the range of applications.
- Polymer identity: for each use of SPM, generic information on the identity of the polymers used should be included. This helps authorities categorize and monitor different types of microparticles.
- Quantity estimates: manufacturers and users must estimate the quantity of SPM released into the environment during the previous calendar year for each end use. This estimate assists in assessing environmental impact.
Reporting obligation is typically required annually, with deadlines for submission set by regulatory authorities (different depending on your company business).
- Starting from 2026 by 31 May of each year for manufacturers and industrial downstream users of SPM in the form of pellets, flakes, and powders
- Starting from 2027 by 31 May of each year for other manufacturers and industrial downstream of SPM at industrial sites.
- Starting from 2027 by 31 May of each year for suppliers of products containing SPM that can be derogated. (end uses, applicable derogation should be submitted)
__ Good to know
Starting from 2025, the suppliers of SPM of excluded product categories are required to inform the downstream user of the presence of microplastics as well as providing appropriate instructions for use according the reporting requirement stating "The synthetic polymer microparticles supplied is subject to conditions laid down by entry 78 of Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council".
The EU Commission's decision to restrict intentional microplastics in cosmetics marks a crucial step toward mitigating the environmental and health risks associated with these particles. By gradually phasing out the use of synthetic polymer microparticles, the EU aims to minimize their release into the environment and reduce their presence in ecosystems and the food chain. The amendment underscores the EU's commitment to sustainable practices in the cosmetics industry and sets an example for other regions to address the issue of microplastics comprehensively.
Would you like to find out more about cosmetics regulations?
Don't hesitate to contact us via our contact form.
_







