
The DGCCRF's assessment of nanomaterials on French cosmetics market
A few weeks ago, the DGCCRF (Direction G�n�rale de la Concurrence, de la Consommation et la R�pression des Fraudes) presented the results of the sampling carried out in 2020 on cosmetic products on the French market, especially regarding nanomaterials. Among the 38 samples (11 ingredients and 27 finished products), the results are not really encouraging and show that the cosmetics industry has much progress to make. The breakdown between compliant and non-compliant products and ingredients was as follows:
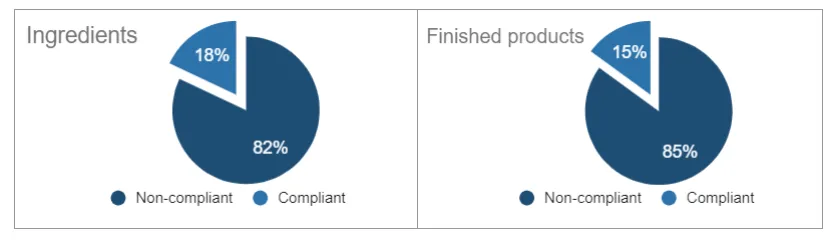
At the level of ingredients, the 82% non-compliant samples were declared as "non-nano" whereas they were indeed identified as nanomaterials by the DGCCRF analysis.
With regard to finished products, among the non-compliant products, the non-compliances were related to the labeling of nanomaterials (especially for UV filters), unauthorized colorings, and other non-compliances with the provisions on nanos in cosmetic products.
Whether in regulatory or health terms, the issue of nanomaterials is therefore complex and impacts industries in many ways. Cosmetics manufacturers, the people responsible for them, as well as ingredient suppliers are indeed impacted by the presence of nanomaterials.
This kind of control pushes brands to be more demanding towards their suppliers on the quality of the ingredients provided as well as on the data made available. This is particularly true in France, which is one of the countries most careful about nanomaterials.
Look back at the European obligations regarding nanomaterials and cosmetics
Nanomaterials are defined by the cosmetic regulation as "an insoluble or biopersistent material, intentionally manufactured and characterized by one or more external dimensions, or an internal structure, on a scale of 1 to 100 nm". The presence of nanomaterials in cosmetics is a major issue: the safety assessment of the product will indeed not be exactly the same depending on whether it contains nanomaterials or not.
In addition to safety assessment, the presence of nanomaterials has regulatory impacts. In particular, responsible persons must:
- notify the use of nanomaterials on the CPNP (Cosmetic Product Notification Portal), when they are used for a function other than coloring, preserving or sunscreening and are not prohibited;
- label the ingredient with the suffix [nano] in the product's ingredient list
- for colorants, preservatives and UV filters, only use nanomaterials explicitly allowed by the cosmetics regulation 1223/2009.
The lack of harmonization between the various European regulations on nanomaterials poses a problem, particularly because of the absence of a threshold, the "intentionality" criterion for considering an ingredient as nano, the diversity of possible analyses, etc.
In addition to the obligations of marketers, ingredient suppliers also have obligations. Indeed, in a joint note, the DGCCRF and the ANSM recall that the cosmetic regulation does not require the ingredient supplier to inform the person responsible for the nano nature of substances incorporated in finished products. However, it is also recalled that the civil code requires the seller to provide his co-contractor with "any information whose importance is decisive for his consent". He must therefore provide all data and characterizations of the nanomaterials obtained.
France has its own nanomaterials register, R-nano, but it only concerns substances. Only suppliers of ingredients, in respect of their substances, are required to declare on this register.
Wish to know more about the compliance of your cosmetic products?

For more information, do not hesitate to contact Nataliya Muller or one of our experts!
The DGCCRF's assessment of nanomaterials on French cosmetics market
A few weeks ago, the DGCCRF (Direction G�n�rale de la Concurrence, de la Consommation et la R�pression des Fraudes) presented the results of the sampling carried out in 2020 on cosmetic products on the French market, especially regarding nanomaterials. Among the 38 samples (11 ingredients and 27 finished products), the results are not really encouraging and show that the cosmetics industry has much progress to make. The breakdown between compliant and non-compliant products and ingredients was as follows:

At the level of ingredients, the 82% non-compliant samples were declared as "non-nano" whereas they were indeed identified as nanomaterials by the DGCCRF analysis.
With regard to finished products, among the non-compliant products, the non-compliances were related to the labeling of nanomaterials (especially for UV filters), unauthorized colorings, and other non-compliances with the provisions on nanos in cosmetic products.
Whether in regulatory or health terms, the issue of nanomaterials is therefore complex and impacts industries in many ways. Cosmetics manufacturers, the people responsible for them, as well as ingredient suppliers are indeed impacted by the presence of nanomaterials.
This kind of control pushes brands to be more demanding towards their suppliers on the quality of the ingredients provided as well as on the data made available. This is particularly true in France, which is one of the countries most careful about nanomaterials.
Look back at the European obligations regarding nanomaterials and cosmetics
Nanomaterials are defined by the cosmetic regulation as "an insoluble or biopersistent material, intentionally manufactured and characterized by one or more external dimensions, or an internal structure, on a scale of 1 to 100 nm". The presence of nanomaterials in cosmetics is a major issue: the safety assessment of the product will indeed not be exactly the same depending on whether it contains nanomaterials or not.
In addition to safety assessment, the presence of nanomaterials has regulatory impacts. In particular, responsible persons must:
- notify the use of nanomaterials on the CPNP (Cosmetic Product Notification Portal), when they are used for a function other than coloring, preserving or sunscreening and are not prohibited;
- label the ingredient with the suffix [nano] in the product's ingredient list
- for colorants, preservatives and UV filters, only use nanomaterials explicitly allowed by the cosmetics regulation 1223/2009.
The lack of harmonization between the various European regulations on nanomaterials poses a problem, particularly because of the absence of a threshold, the "intentionality" criterion for considering an ingredient as nano, the diversity of possible analyses, etc.
In addition to the obligations of marketers, ingredient suppliers also have obligations. Indeed, in a joint note, the DGCCRF and the ANSM recall that the cosmetic regulation does not require the ingredient supplier to inform the person responsible for the nano nature of substances incorporated in finished products. However, it is also recalled that the civil code requires the seller to provide his co-contractor with "any information whose importance is decisive for his consent". He must therefore provide all data and characterizations of the nanomaterials obtained.
France has its own nanomaterials register, R-nano, but it only concerns substances. Only suppliers of ingredients, in respect of their substances, are required to declare on this register.
Wish to know more about the compliance of your cosmetic products?

For more information, do not hesitate to contact Nataliya Muller or one of our experts!




.png)


