What is REACH authorisation ?
REACH regulation establishes annexes with substances subjected to different use rules. Annex XIV or “list of substances subject to authorisation” regroups substances with severe or proven hazards for human health and/or the environment, and for which the use will be banned from a certain date, unless a temporary use authorisation has been granted by ECHA following the submission of an authorisation dossier.
ECHA’s Risk Assessment Committees (RAC) and Socio-Economic Analysis Committee (SEAC) give opinions on the latter, which are taken into account in the European Commission’s final decision. The granted authorisation represents a temporary derogation with the aim for the industry or manufacturer to substitute the substance.
REACH authorisation: a procedure criticised by NGO ChemSec
In its 2016 annual report published last May, ECHA says it received 77 applications for 112 uses of SVHCs (Substances of Very High Concern), almost double its estimated number of 60.
This number is too high according to the non-governmental organisation, ChemSec, who works towards finding alternatives to hazardous substances for companies to substitute these substances.
The NGO reminds that the first aim of the Authorisation list (or Annex XIV of REACH) is to encourage the industry to find more viable alternatives and to draw attention on the contradictory character of the decision to attribute so many temporary use authorisations and to encourage companies to use other substances. “Some companies have worked hard to phase out substances and use alternatives, while others get authorisation to continue using those substances for another 12 years.” says Frida Hök from ChemSec.
This could hinder innovation and minimise the impact of the REACH regulation, according to ChemSec.
The non-profit organisation also criticises ECHA’s operation and questions why there is no committee dedicated to assessing the alternatives in the Member States Committee. As a matter of fact : - the Risk Assessment Committee (RAC) prepares the opinions on the risks of the substances for human health and the environment within the REACH and CLP regulation – the Socio-Economic Committee (SEAC) prepares ECHA’s opinions on the potential socio-economic impact of regulatory measures on chemical substances. The substitution analysis is conducted through peer review during the 2 months of public consultation of the dossier.
ChemSec : an NGO specialised in hazardous substance substitution
ChemSec strives to raise public awareness on hazardous substance substitution through:
- Webinars
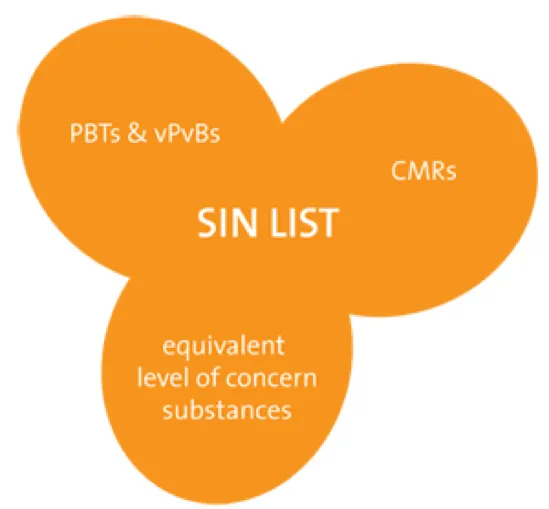
- The SIN List (Substitute It Now! List): a list of substances that aren’t classified as SVHCs but that present possibly hazardous properties. This list was created and is regularly updated by ChemSec, and is for the industry that uses chemical substances.
- More recently the creation of Marketplace, a web platform where buyers and sellers of alternatives to hazardous chemicals can interact
Its mission: to bridge the gap between decision-makers, industries, non-profit organisations and scientists.
Through campaigns and legislative processes, ChemSec’s work is based on four principles: the Precautionary principle, the Substitution principle, the Polluter pays principle, and the Right to Know principle. To learn more, go to their website.
Created in 2002, today this NGO list 912 substances on the « SIN List » and makes chemical management tools available. It strives for a toxic free environment.
ECHA claims a flexible and reasoned strategy
The aim of the Authorisation procedure is to guarantee risk management for the use of SVHCs and their progressive substitution, whatever the tonnage. In this sense, Authorisation is a transition bridge for the industry towards substitution through safer alternatives all the while being flexible concerning the complete and immediate ban. ECHA reminds that authorisation is only granted when a viable substitution plan is presented in the dossier.
Its statistics show 111 authorisation applications received over the last five years (From 2012 to 30 January 2017). These authorisation applications represent 195 applicants for 180 uses of SVHC substances.
In the 2016 general report, Echa head Geert Dancet says that the authorisation process has matured and become clearer. The increasingly efficient authorisation system has encouraged more companies to take innovative approaches to finding safer alternatives. The processes are regularly optimised thanks to the efforts of the Agency’s work teams.
Moreover, the temporary opinion expressed following the different assessments, is submitted to the authorisation applicant to take into account any potential comments.
Temporary authorisation as opposed to a ban means more responsibility and exchange between companies to reach collaborative decisions and the most appropriate for each case. The aim isn’t to turn a blind eye on uses that are at risk but to assist manufacturers and help them find the best alternative, in keeping with their economic constraints. The approach is more flexible" to have a realistic management of the challenges.
Authorisation is granted for a given period during which the manufacturers /users commit to implement a substitution plan. You can find the process below:
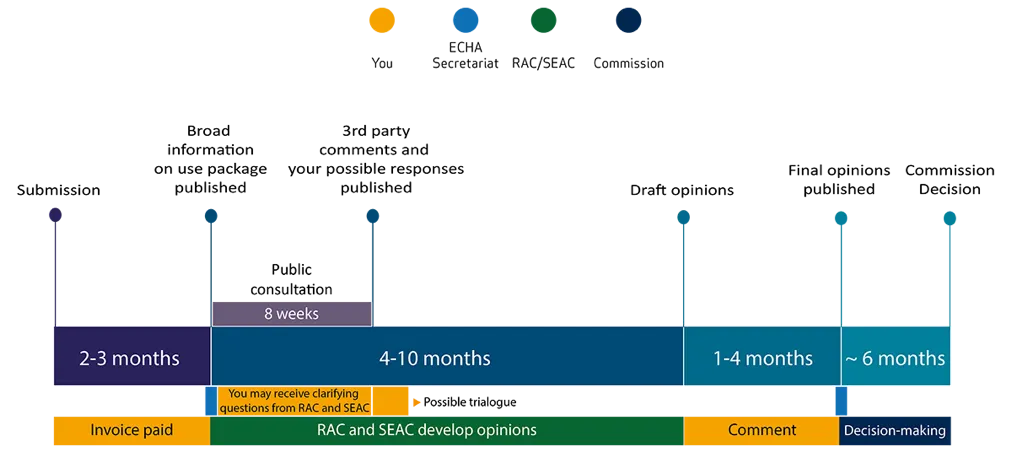
Source : ECHA
Note that the granting of the authorisation depends on the quality of the Analysis of Alternatives of the dossier’s hazardous substance. This analysis requires a reasoned substitution plan, which is subject to peer review during the dossier submission.
Good Practices for a convincing Analysis of Alternatives
The methodology recommended and applied by EcoMundo in terms of Analysis of Alternatives (AoA) in an Authorisation dossier aims to capitalise on the experience and expertise of the company submitting the dossier. The description of the substitution process foreseen by the company relies to a great extent on the expertise of internal expert resources (R&D, innovation) to demonstrate the unavailability of alternatives.
EcoMundo establishes the technical reasoning, in order to meet ECHA’s requirements in terms of structure and level of detail. The information provided by the company is systematically assessed with regards to scientific publications or expert opinions to guarantee their robustness. This means that the AoA is not only organised around generic alternatives research but also on client technology and thus constitutes a tailored alternatives research.
This methodology has already been proven as 100% of Authorisation applications submitted since 2015 by EcoMundo received the exact durations of review period requested by our clients, including for « long-term » durations of 12 years. As opposed to the EU average where more than half of the dossiers submitted to ECHA to this day received reduced Authorisation durations compared to what they asked for.
How to anticipate the hazardous substances to substitute?
To avoid having to substitute or apply for authorisation, the best solution is to anticipate, before the product’s design, which substances would be wiser to use and which ones would possibly enter Annex XIV of REACH.
Other than the assistance offered for the REACH authorisation dossier creation, EcoMundo also helps its clients with the product portfolio securing, by identifying the substances presenting a risk of being subject to future regulatory restrictions or by assisting its clients during the public consultation stages before a substance is regulated.
Such an approach is a real key to securing the industrial activity.
Authorisation Services & Substances Securing
EcoMundo’s experts are there to answer your questions and assist you in the different stages of the REACH procedures:
- Conduct an Authorisation or Defense exemption diagnostic
- Individually conduct an Authorisation dossier
- Collectively conduct an Authorisation dossier
- Comply with the Authorisation requirements
- Anticipate the regulatory challenges of your substances used in R&D
- Shape your substitution strategy for Annex XIV substances
- Find out the toxicological and regulatory status of these substances
- Identify the substances presenting a future regulatory risk
- Find out the breakdown of your materials into substances
What is REACH authorisation ?
REACH regulation establishes annexes with substances subjected to different use rules. Annex XIV or “list of substances subject to authorisation” regroups substances with severe or proven hazards for human health and/or the environment, and for which the use will be banned from a certain date, unless a temporary use authorisation has been granted by ECHA following the submission of an authorisation dossier.
ECHA’s Risk Assessment Committees (RAC) and Socio-Economic Analysis Committee (SEAC) give opinions on the latter, which are taken into account in the European Commission’s final decision. The granted authorisation represents a temporary derogation with the aim for the industry or manufacturer to substitute the substance.
REACH authorisation: a procedure criticised by NGO ChemSec
In its 2016 annual report published last May, ECHA says it received 77 applications for 112 uses of SVHCs (Substances of Very High Concern), almost double its estimated number of 60.
This number is too high according to the non-governmental organisation, ChemSec, who works towards finding alternatives to hazardous substances for companies to substitute these substances.
The NGO reminds that the first aim of the Authorisation list (or Annex XIV of REACH) is to encourage the industry to find more viable alternatives and to draw attention on the contradictory character of the decision to attribute so many temporary use authorisations and to encourage companies to use other substances. “Some companies have worked hard to phase out substances and use alternatives, while others get authorisation to continue using those substances for another 12 years.” says Frida Hök from ChemSec.
This could hinder innovation and minimise the impact of the REACH regulation, according to ChemSec.
The non-profit organisation also criticises ECHA’s operation and questions why there is no committee dedicated to assessing the alternatives in the Member States Committee. As a matter of fact : - the Risk Assessment Committee (RAC) prepares the opinions on the risks of the substances for human health and the environment within the REACH and CLP regulation – the Socio-Economic Committee (SEAC) prepares ECHA’s opinions on the potential socio-economic impact of regulatory measures on chemical substances. The substitution analysis is conducted through peer review during the 2 months of public consultation of the dossier.
ChemSec : an NGO specialised in hazardous substance substitution
ChemSec strives to raise public awareness on hazardous substance substitution through:
- Webinars
- The SIN List (Substitute It Now! List): a list of substances that aren’t classified as SVHCs but that present possibly hazardous properties. This list was created and is regularly updated by ChemSec, and is for the industry that uses chemical substances.
- More recently the creation of Marketplace, a web platform where buyers and sellers of alternatives to hazardous chemicals can interact

Its mission: to bridge the gap between decision-makers, industries, non-profit organisations and scientists.
Through campaigns and legislative processes, ChemSec’s work is based on four principles: the Precautionary principle, the Substitution principle, the Polluter pays principle, and the Right to Know principle. To learn more, go to their website.
Created in 2002, today this NGO list 912 substances on the « SIN List » and makes chemical management tools available. It strives for a toxic free environment.
ECHA claims a flexible and reasoned strategy
The aim of the Authorisation procedure is to guarantee risk management for the use of SVHCs and their progressive substitution, whatever the tonnage. In this sense, Authorisation is a transition bridge for the industry towards substitution through safer alternatives all the while being flexible concerning the complete and immediate ban. ECHA reminds that authorisation is only granted when a viable substitution plan is presented in the dossier.
Its statistics show 111 authorisation applications received over the last five years (From 2012 to 30 January 2017). These authorisation applications represent 195 applicants for 180 uses of SVHC substances.
In the 2016 general report, Echa head Geert Dancet says that the authorisation process has matured and become clearer. The increasingly efficient authorisation system has encouraged more companies to take innovative approaches to finding safer alternatives. The processes are regularly optimised thanks to the efforts of the Agency’s work teams.
Moreover, the temporary opinion expressed following the different assessments, is submitted to the authorisation applicant to take into account any potential comments.
Temporary authorisation as opposed to a ban means more responsibility and exchange between companies to reach collaborative decisions and the most appropriate for each case. The aim isn’t to turn a blind eye on uses that are at risk but to assist manufacturers and help them find the best alternative, in keeping with their economic constraints. The approach is more flexible" to have a realistic management of the challenges.
Authorisation is granted for a given period during which the manufacturers /users commit to implement a substitution plan. You can find the process below:

Source : ECHA
Note that the granting of the authorisation depends on the quality of the Analysis of Alternatives of the dossier’s hazardous substance. This analysis requires a reasoned substitution plan, which is subject to peer review during the dossier submission.
Good Practices for a convincing Analysis of Alternatives
The methodology recommended and applied by EcoMundo in terms of Analysis of Alternatives (AoA) in an Authorisation dossier aims to capitalise on the experience and expertise of the company submitting the dossier. The description of the substitution process foreseen by the company relies to a great extent on the expertise of internal expert resources (R&D, innovation) to demonstrate the unavailability of alternatives.
EcoMundo establishes the technical reasoning, in order to meet ECHA’s requirements in terms of structure and level of detail. The information provided by the company is systematically assessed with regards to scientific publications or expert opinions to guarantee their robustness. This means that the AoA is not only organised around generic alternatives research but also on client technology and thus constitutes a tailored alternatives research.
This methodology has already been proven as 100% of Authorisation applications submitted since 2015 by EcoMundo received the exact durations of review period requested by our clients, including for « long-term » durations of 12 years. As opposed to the EU average where more than half of the dossiers submitted to ECHA to this day received reduced Authorisation durations compared to what they asked for.
How to anticipate the hazardous substances to substitute?
To avoid having to substitute or apply for authorisation, the best solution is to anticipate, before the product’s design, which substances would be wiser to use and which ones would possibly enter Annex XIV of REACH.
Other than the assistance offered for the REACH authorisation dossier creation, EcoMundo also helps its clients with the product portfolio securing, by identifying the substances presenting a risk of being subject to future regulatory restrictions or by assisting its clients during the public consultation stages before a substance is regulated.
Such an approach is a real key to securing the industrial activity.
Authorisation Services & Substances Securing
EcoMundo’s experts are there to answer your questions and assist you in the different stages of the REACH procedures:
- Conduct an Authorisation or Defense exemption diagnostic
- Individually conduct an Authorisation dossier
- Collectively conduct an Authorisation dossier
- Comply with the Authorisation requirements
- Anticipate the regulatory challenges of your substances used in R&D
- Shape your substitution strategy for Annex XIV substances
- Find out the toxicological and regulatory status of these substances
- Identify the substances presenting a future regulatory risk
- Find out the breakdown of your materials into substances







