Should you re-certify your medical devices?
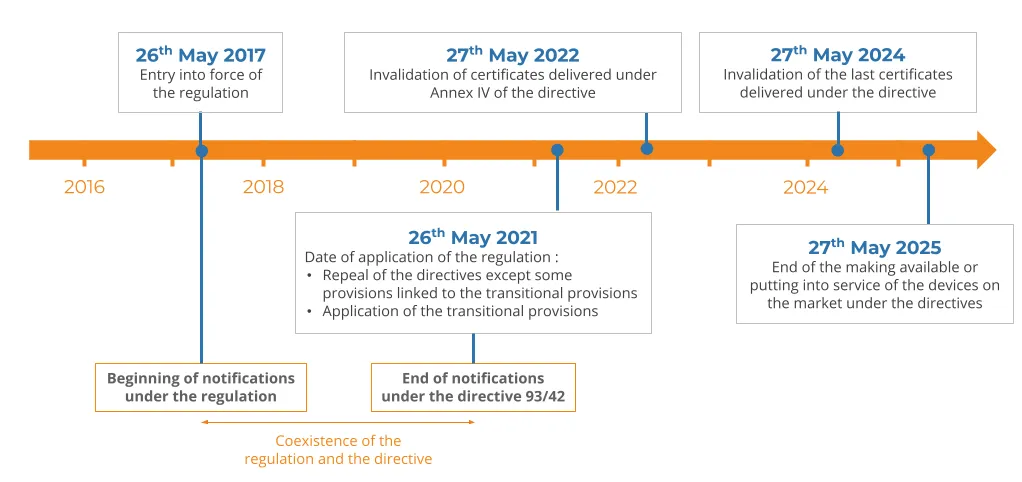
In May 2021, the Regulation (EU) 2017/745 relating to medical devices will start applying. Medical devices will therefore need to be brought into compliance with this new regulation, bit by bit, depending on the devices� class and date of putting on the market.
As for certifications, every new medical device will need to be certified under the new regulation starting on the implementation date in May. Medical devices which were marketed before this date will ultimately need to be notified under the new regulation.
To this end, the deadlines are as follow:
- At its date of application, in May 2021, all classes of devices will need to comply with the regulation,
- Between 2022 and 2024, certificates delivered under the directives will be invalidated,
- On 27th May 2024, with the invalidation of the last certificates, all medical devices from class IIa to III will have to comply with the regulation,
- Devices legally commercialised under the directives may still be made available on the market or put into service until 27th May 2025.
All medical devices will thus need to be notified under the new regulation, first the new ones then, little by little, the old ones too.
The regulation on medical devices
The regulation on medical devices, which entered into force in 2017, replaces and repeals the old directives which, until then, ruled medical devices in the European Union.
A regulation, unlike a directive, is directly applicable in all Member States, with no room for interpretation and no requirement to be transposed into national law.
This regulation harmonises the legislation on medical devices, while widening the application scope, adding new classification rules, and improving the surveillance before commercialisation and post-marketing monitoring.
Wish to know more about medical devices?

For more information, do not hesitate to contact Ra�ssa Abdel Kader or one of our experts!
Should you re-certify your medical devices?
In May 2021, the Regulation (EU) 2017/745 relating to medical devices will start applying. Medical devices will therefore need to be brought into compliance with this new regulation, bit by bit, depending on the devices� class and date of putting on the market.
As for certifications, every new medical device will need to be certified under the new regulation starting on the implementation date in May. Medical devices which were marketed before this date will ultimately need to be notified under the new regulation.
To this end, the deadlines are as follow:
- At its date of application, in May 2021, all classes of devices will need to comply with the regulation,
- Between 2022 and 2024, certificates delivered under the directives will be invalidated,
- On 27th May 2024, with the invalidation of the last certificates, all medical devices from class IIa to III will have to comply with the regulation,
- Devices legally commercialised under the directives may still be made available on the market or put into service until 27th May 2025.
All medical devices will thus need to be notified under the new regulation, first the new ones then, little by little, the old ones too.

The regulation on medical devices
The regulation on medical devices, which entered into force in 2017, replaces and repeals the old directives which, until then, ruled medical devices in the European Union.
A regulation, unlike a directive, is directly applicable in all Member States, with no room for interpretation and no requirement to be transposed into national law.
This regulation harmonises the legislation on medical devices, while widening the application scope, adding new classification rules, and improving the surveillance before commercialisation and post-marketing monitoring.
Wish to know more about medical devices?

For more information, do not hesitate to contact Ra�ssa Abdel Kader or one of our experts!