
What is the "same" biocidal product MA?
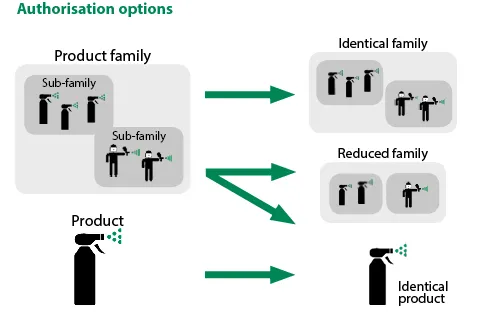
The “same” product Marketing Authorisation (MA) allows companies to go from wider to narrower authorisations. That is to say, from a biocidal product family to a subset of one or more “family members” or from wider to narrower markets (from Union authorisation to national authorisation).
Thus, it is possible to request an authorisation for a product if the latter is identical to another authorised biocidal product (reference product) or to another product for which an authorisation request has been submitted (the future reference product).
Nonetheless, both products have to have the same composition and uses. Moreover, the reference product needs to possess a national MA or a Union one (or currently be under evaluation to get it) in order to be able to request a same product MA.
Source : ECHA
For more information, you can take a look at the regulation dealing with same biocidal product MAs.
Dossier submission
According to the Biocidal Product Regulation (BPR), all requests for same biocidal product MA have to be submitted via the R4BP platform, as well as all other MA requests.
In order to be able to benefit from the transitory period, you have to submit your dossier before the approbation date of your substance; just like every other long-term MA request.
The evaluation process
After passing the first round of tests performed by ECHA, the dossier is transferred to the competent authority for validation. It is the latter who will take the final decision.
For more information on the procedure for same product Marketing Authorisation, do not hesitate to visit ECHA’s website and the practical guide on same biocidal product MAs.
Supporting documents
ANSES’s biocides helpdesk gathers the list of all the supporting documents you need for your same product authorisation request. Click here to see it.
Linked fees
The fees linked to national authorisation may vary according to Member States. Therefore, it is the applicant’s responsibility to enquire about this issue to the competent evaluating authority.
What is the "same" biocidal product MA?
The “same” product Marketing Authorisation (MA) allows companies to go from wider to narrower authorisations. That is to say, from a biocidal product family to a subset of one or more “family members” or from wider to narrower markets (from Union authorisation to national authorisation).
Thus, it is possible to request an authorisation for a product if the latter is identical to another authorised biocidal product (reference product) or to another product for which an authorisation request has been submitted (the future reference product).
Nonetheless, both products have to have the same composition and uses. Moreover, the reference product needs to possess a national MA or a Union one (or currently be under evaluation to get it) in order to be able to request a same product MA.

Source : ECHA
For more information, you can take a look at the regulation dealing with same biocidal product MAs.
Dossier submission
According to the Biocidal Product Regulation (BPR), all requests for same biocidal product MA have to be submitted via the R4BP platform, as well as all other MA requests.
In order to be able to benefit from the transitory period, you have to submit your dossier before the approbation date of your substance; just like every other long-term MA request.
The evaluation process
After passing the first round of tests performed by ECHA, the dossier is transferred to the competent authority for validation. It is the latter who will take the final decision.
For more information on the procedure for same product Marketing Authorisation, do not hesitate to visit ECHA’s website and the practical guide on same biocidal product MAs.
Supporting documents
ANSES’s biocides helpdesk gathers the list of all the supporting documents you need for your same product authorisation request. Click here to see it.
Linked fees
The fees linked to national authorisation may vary according to Member States. Therefore, it is the applicant’s responsibility to enquire about this issue to the competent evaluating authority.







