.png)
What is an endocrine disruptor?
An endocrine disruptor is a substance (chemical, natural, drug,...) that can interfere with the body's endocrine system. These substances interfere with natural hormones that regulate functions such as growth and development, metabolism and reproduction by three main mechanisms.
Endocrine disruptors can mimic, block or disrupt the production, transport, elimination or regulation of natural hormones which can lead to adverse health effects such as developmental abnormalities, cancers and reproductive problems.
New hazard classes for Endocrine Disruptors, PBT and PMT substances: What you need to know
Since 2018, it has been mandatory to evaluate substances for their potential to cause Endocrine Disruption (ED) under the Plant Protection Products and Biocide Products regulations.
The year 2023 is a crucial moment as the CLP regulation has introduced new hazard classes for ED, which were initially introduced in December 2022 with an effective date of 20 April 2023:
- ED HH in Category 1 and Category 2 (Endocrine disruption for human health)
- ED ENV in Category 1 and Category 2 (Endocrine disruption for the environment)
Category 1: Known or presumed endocrine disruptors for human health and/or for the environment. For this category, the limit concentration is 0,1% in the mixture. This implies new hazard statements:
- EUH380 for human health: May cause endocrine disruption in humans
- EUH430 for the environment: May cause endocrine disruption in the environment
Category 2: Suspected endocrine disruptors for human health and/or for the environment. For this category, the limit concentration is 1% in the mixture. The relevant new hazard statements are:
- EUH381 for human health: Suspected of causing endocrine disruption in humans
- EUH431 for environment: Suspected of causing endocrine disruption in the environment
Furthermore, other substances are classified as PBT (persistent, bioaccumulative, toxic), vPvB (very persistent, very bioaccumulative), PMT (persistent, mobile, toxic), and vPvM (very persistent, very mobile). The mixture is classified when at least one compound is classified and is present at 0.1%. This also implies new hazard statements accordingly:
- For PBT: EUH440: Accumulates in the environment and in living organisms including in humans
- For vPvB: EUH441: Strongly accumulates in the environment and living organisms including in humans
- For PMT: EUH450: Can cause long-lasting and diffuse contamination of water resources
- For vPvM: EUH451: Can cause very long-lasting and diffuse contamination of water resources
Manufacturers, importers, downstream users, and distributors placing substances on the European Union market are bound by this EU legislation. Member States will also refer to the new hazard classes and criteria when making proposals for harmonized classification and labelling.
Guidance for companies placing products on the market containing EDs, PMTs and PBTs to ensure compliance and avoid penalties
From April 20th 2023, Member States can propose harmonized classification and labelling with the new hazard classes, and manufacturers, importers, downstream users, and distributors can self-classify their substances and mixtures accordingly. However, there are transitional periods, during which the new hazard classes are not yet mandatory but based on voluntary action.
For new substances on the market, companies need to comply with the new rules from May 1st 2025, while substances that have already been on the EU market must comply by November 1st 2026. Separate transition times apply for mixtures, with new hazard classes applying from May 1st 2026 for new mixtures, and companies having until 1 May 2028 to update the classification and labeling for existing mixtures on the EU market.
Furthermore, the update of regulations on Cosmetic Products to take into account ED assessment is expected in the forthcoming months, and the next update of the REACH regulation is anticipated to include new requirements to identify ED from the lowest tonnage band by the end of this year or the beginning of the next.
Check out our timeline on Substances:
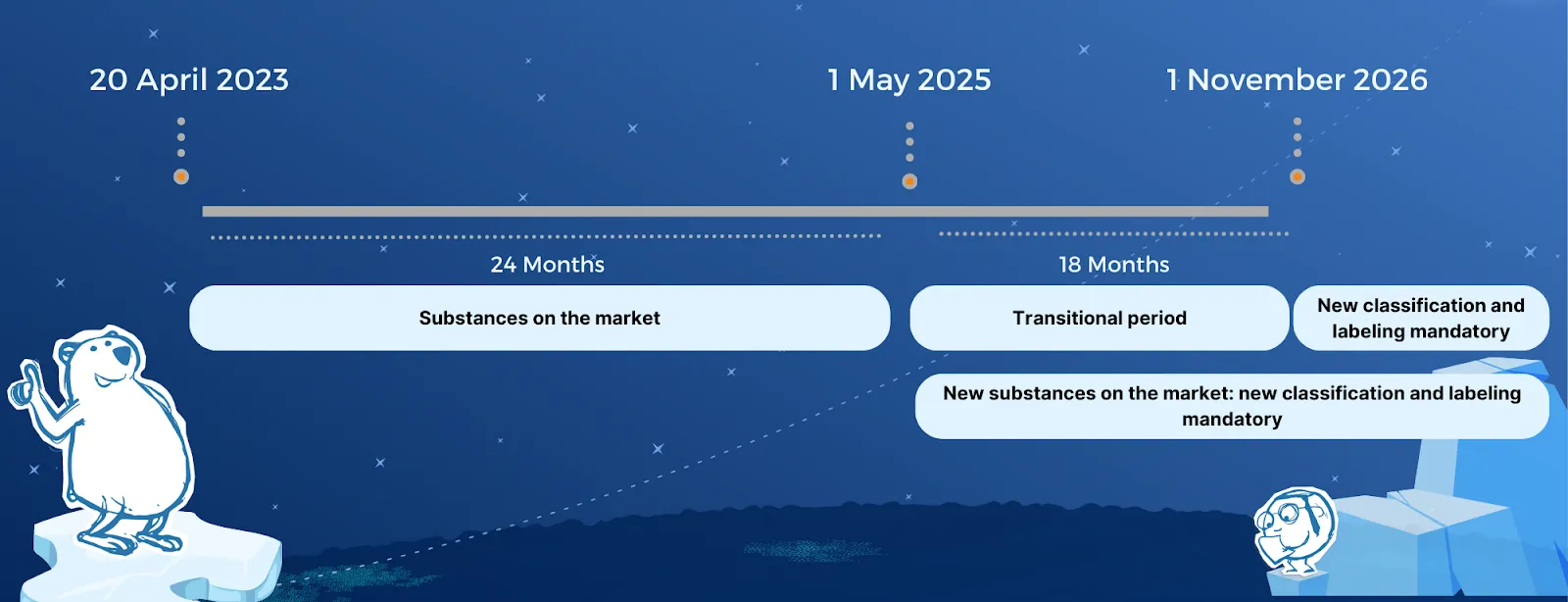
Mixtures:
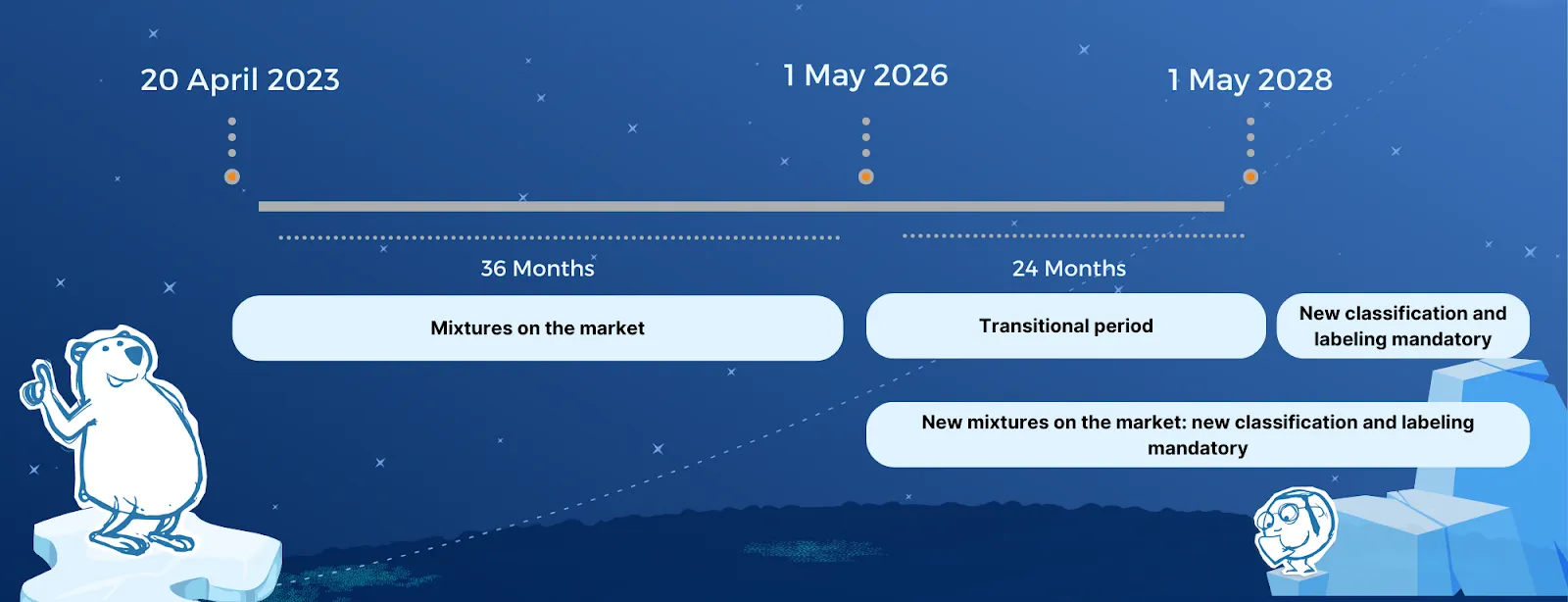
EcoMundo's support
Take action now to ensure accurate classification, labeling, and packaging of your products! If you require assistance with endocrine disruptor (ED), persistent, bioaccumulative, and toxic (PBT), or very persistent and very bioaccumulative (PMT) assessments, we are here to help.
Our team can review the formulas of your products and update their classifications based on the presence of ED/PBT/PMT substances. Don't compromise on compliance contact us today to verify your classifications and make necessary updates.
What is an endocrine disruptor?
An endocrine disruptor is a substance (chemical, natural, drug,...) that can interfere with the body's endocrine system. These substances interfere with natural hormones that regulate functions such as growth and development, metabolism and reproduction by three main mechanisms.
Endocrine disruptors can mimic, block or disrupt the production, transport, elimination or regulation of natural hormones which can lead to adverse health effects such as developmental abnormalities, cancers and reproductive problems.
New hazard classes for Endocrine Disruptors, PBT and PMT substances: What you need to know
Since 2018, it has been mandatory to evaluate substances for their potential to cause Endocrine Disruption (ED) under the Plant Protection Products and Biocide Products regulations.
The year 2023 is a crucial moment as the CLP regulation has introduced new hazard classes for ED, which were initially introduced in December 2022 with an effective date of 20 April 2023:
- ED HH in Category 1 and Category 2 (Endocrine disruption for human health)
- ED ENV in Category 1 and Category 2 (Endocrine disruption for the environment)
Category 1: Known or presumed endocrine disruptors for human health and/or for the environment. For this category, the limit concentration is 0,1% in the mixture. This implies new hazard statements:
- EUH380 for human health: May cause endocrine disruption in humans
- EUH430 for the environment: May cause endocrine disruption in the environment
Category 2: Suspected endocrine disruptors for human health and/or for the environment. For this category, the limit concentration is 1% in the mixture. The relevant new hazard statements are:
- EUH381 for human health: Suspected of causing endocrine disruption in humans
- EUH431 for environment: Suspected of causing endocrine disruption in the environment
Furthermore, other substances are classified as PBT (persistent, bioaccumulative, toxic), vPvB (very persistent, very bioaccumulative), PMT (persistent, mobile, toxic), and vPvM (very persistent, very mobile). The mixture is classified when at least one compound is classified and is present at 0.1%. This also implies new hazard statements accordingly:
- For PBT: EUH440: Accumulates in the environment and in living organisms including in humans
- For vPvB: EUH441: Strongly accumulates in the environment and living organisms including in humans
- For PMT: EUH450: Can cause long-lasting and diffuse contamination of water resources
- For vPvM: EUH451: Can cause very long-lasting and diffuse contamination of water resources
Manufacturers, importers, downstream users, and distributors placing substances on the European Union market are bound by this EU legislation. Member States will also refer to the new hazard classes and criteria when making proposals for harmonized classification and labelling.
Guidance for companies placing products on the market containing EDs, PMTs and PBTs to ensure compliance and avoid penalties
From April 20th 2023, Member States can propose harmonized classification and labelling with the new hazard classes, and manufacturers, importers, downstream users, and distributors can self-classify their substances and mixtures accordingly. However, there are transitional periods, during which the new hazard classes are not yet mandatory but based on voluntary action.
For new substances on the market, companies need to comply with the new rules from May 1st 2025, while substances that have already been on the EU market must comply by November 1st 2026. Separate transition times apply for mixtures, with new hazard classes applying from May 1st 2026 for new mixtures, and companies having until 1 May 2028 to update the classification and labeling for existing mixtures on the EU market.
Furthermore, the update of regulations on Cosmetic Products to take into account ED assessment is expected in the forthcoming months, and the next update of the REACH regulation is anticipated to include new requirements to identify ED from the lowest tonnage band by the end of this year or the beginning of the next.
Check out our timeline on Substances:

Mixtures:

EcoMundo's support
Take action now to ensure accurate classification, labeling, and packaging of your products! If you require assistance with endocrine disruptor (ED), persistent, bioaccumulative, and toxic (PBT), or very persistent and very bioaccumulative (PMT) assessments, we are here to help.
Our team can review the formulas of your products and update their classifications based on the presence of ED/PBT/PMT substances. Don't compromise on compliance contact us today to verify your classifications and make necessary updates.







