
What is a Responsible Person?
The European regulation EC No. 1223/2009 defines the Responsible Person (RP) in the article 4 as a legal or natural person based in the European Union who will act as the unique representative throughout the EU. The RP’s role is to ensure that each cosmetic product personal care product marketed in the EU countries complies with the Cosmetics Regulation 1223/2009 which aims to establish that the cosmetic product is safe for use.
Who can be a Responsible Person?
The Responsible Person may be a manufacturer, importer or distributor, but also a consultant likes EcoMundo. Usually, the one who places the product on the EU market will be the one who will ensure this role unless a third person is designated. Nevertheless you have to keep in mind that only a legal or natural person based in the EU can ensure this role.
A- The manufacturer
The Cosmetics Regulation defines the manufacturer as “any natural or legal person who manufactures a cosmetic product or has such a product designed or manufactured, and markets that cosmetic product under his name or trademark”. »
The manufacturer established within the EU is the Responsible Person for the cosmetic products he manufactures (in the EU) if they are not subject to be exported and reimported into the EU.
The manufacturer may also designate any person established in the community by mandating someone with a written letter. The designated person may accept the role by responding in writing.
B- The importer
Defined by article 2 of the Cosmetics Regulation an importer is “any natural or legal person established within the Community, who places a cosmetic product from a third country on the Community market”. »
C- The distributor
The distributor is defined by the EU Regulation No.1223/2009 as “any natural or legal person in the supply chain, other than the manufacturer or the importer, who makes a cosmetic product available on the community market” »
The distributor is the Responsible Person if the product is placed on the market under his name or trademark or if he modifies a product already on the market and takes the risk to affect the compliance to the Cosmetics Regulation.
D- Any person established in the European Union
The manufacturer or the importer can designate a third person established in the EU to be a Responsible Person.
In this case the only formality required by the Cosmetics Regulation is a written agreement. In other words, you must designate your Responsible Person via a written mandate and the RP must accept his role in writing.
The designated RP can be any legal or natural person who is able to endorse the responsibilities of the RP and comply with the regulatory requirements, e.g. such as a regulatory expert like EcoMundo.
Is it mandatory to have a Responsible Person?
Article 4 of the Cosmetics Regulation establishes that only cosmetic products with an appointed Responsible Person may be placed on the market. In other words: a Responsible Person is essential to market your cosmetics.
Therefore, the Responsible Person has many obligations and responsibilities, especially to:
- Ensure that the cosmetic product placed on the market is safe for human health
- Ensure compliance with Cosmetics Regulation obligations for each cosmetic product placed on the market
What are the obligations of the Responsible Person?
The Responsible Person is in charge of ensuring compliance of the cosmetic product with Regulation 1223/2009 and its amendments.
The RP plays an essential role in the marketing process of a cosmetic product.
If you’re a Responsible Person, you have to endorse all these obligations:
1. The Responsible Person guarantees the safety of the cosmetic product
A- Safety of the product made available on the market (Article 3)
The RP must ensure that the cosmetic product complies with Article 3 of Cosmetics Regulation 1223/2009, that is to say, a “cosmetic product made available on the market shall be safe for human health when used under normal or reasonably foreseeable conditions of use.” ».
B- The cosmetic product safety assessment (Article 10)
The Responsible Person ensures that product safety is assessed on the basis of relevant information, and that the safety assessment of the cosmetic product is established under annex 1 of regulation 1223/2009. The Responsible Person may also incorporate information or indications concerning the security in the product safety report.
Article 10 of Cosmetics Regulation states: “prior to placing a cosmetic product on the market, (the RP must) ensure that the cosmetic product has undergone a safety assessment on the basis of the relevant information and that a cosmetic product safety report is set up in accordance with Annex 1” »
According to this article, the Responsible Person must ensure that:
- The intended use of the cosmetic product and the anticipated systemic exposure to individual ingredients in a final formation are taken into account in the safety assessment
- An appropriate weight-of-evidence approach is used in the safety assessment for reviewing data from all existing sources
- The cosmetic product safety report is kept up to date in view of additional relevant information generated after placing the product on the market.
2- The Responsible Person is in charge of the Product Information File of the cosmetic product
The Product Information File (PIF) must be drafted for each cosmetic product put on the European market.
The Responsible Person guarantees that any product placed on the market is linked to a relevant and complete PIF.
The Responsible Person must keep the Product Information File for a period of 10 years after placing the last batch on the market. The Responsible Person must ensure that the competent authority of the Member State which houses the PIF can access that file easily (electronic or paper), at the location indicated on the label.
3- The Responsible Person ensures the quality of the manufacturing process
a) Good Manufacturing Practice (Article 8)
Good Manufacturing Practices (GMP) are issued from the ISO 22716 guideline published in 2007 which provides guidelines for the production, control, storage and shipment of cosmetic products.
The Responsible Person must ensure the compliance with GMP according to Article 8 of the Cosmetics Regulation. The compliance declaration must be included in the PIF of each cosmetic product.

b) Ensure the compliance of sampling and analysis of cosmetic products (Article 12)
Article 12 of the Cosmetics Regulation states that: “Sampling and analysis of cosmetic products shall be performed in a reliable and reproducible manner.” »
Therefore, the RP must ensure the compliance of the sampling process and the analysis of the cosmetic products in accordance to Article 12 of Cosmetics Regulation 1223/2009 and also make sure these analyses and samples are reliable and reproducible. In addition the Cosmetics Regulation states: “reliability and reproducibility shall be presumed if the method used is in accordance with the relevant harmonized standards, the references of which have been published in the Official Journal of European Union.”.
4- The Responsible Person guarantees the compliance of the cosmetic formula
The Responsible Person, as guarantor of the product’s safety, must verify that the formula meets the Cosmetics Regulation’s requirements. Indeed, some cosmetic ingredients are allowed but some are restricted or prohibited by the Annexes of the Cosmetic Regulation. You must ensure that the product composition placed on the market complies with the restrictions of the Cosmetics regulation.
Moreover, the formula must not contain any prohibited substances unless it is a non-intended trace of a prohibited substance, which is technically unavoidable (articles 14, 15, 16, 17 and 24 of 1223/2009 regulation), i.e. incidental ingredients and impurities.
What are the critical regulatory statuses?
The EU Cosmetic Regulation 1223/2009 features several annexes that list some of the regulated substances susceptible to be harmful to human health. There are three categories of ingredients:
a) Prohibited ingredients
Substances in Annex II:
The prohibited substances are classified in annex II of Cosmetic Regulation 1223/2009.
The CMR substances:

The CMR substances (Carcinogenic, mutagenic or toxic to reproduction) are listed in Annex VI of the CLP Regulation (EC No. 1272/2008), and when they are of 2, 1A, 1B categories: they are prohibited.
They may be authorized under certain conditions:
- If they are compliant with the provisions related to the safety of products, as defined by Regulation (EU) No 178/2002
- In case there is no appropriate substitute substance after the analysis of alternative solutions.
- If a request has been made for a particular use of the products’ category with a determined exposure
- When the substances have been assessed and deemed safe by the SCCS (Scientific Committee on Consumer Safety) for use in cosmetic products (sole condition for category CMR2)
b) Restricted substances
These substances are listed in Annex III of the Cosmetics Regulation, which add up to about 300 substances. These ingredients can be used in cosmetics products only under the conditions described in the annex i.e. product type, purity criteria, percentage of maximal use, etc.
c) Allowed substances
Three functions of ingredients are listed by the Regulation: coloring agents, preservatives and UV filters.
- Allowed coloring agents; they are listed in Annex IV of the Cosmetics Regulation (153 of them)
- Allowed preservatives; they are listed in Annex V (59 of them)
- Allowed UV filters: ; they are listed in Annex VI (29 of them)
These ingredients are authorized if they respect the regulation’s restriction i.e. depending on the type of products in which they are contained, the part of the body that is concerned, as well as the concentration of the ingredient in the product.
The specific case of nanomaterials (Article 16)
If you use a nanomaterial that is not listed in Regulation 1223/2009 annexes, the Responsible Person takes on the responsibility to notify the presence of the nanomaterial at least 6 months before the cosmetic product is placed on the market. The notification must be done via the CPNP portal.
The importance of regulatory watch
To check the regulatory compliance of cosmetic formulas, the Responsible Person must analyze all Annexes and compare the ingredients in the composition of the cosmetic product. It is important to note that this work should be done regularly as theses Annexes are updated several times a year (on average every three months).
Depending on the complexity of your formulas, it may be more relevant for the Responsible Person to be assisted by a software such as COSMETIC Factory
5- The Responsible Person checks the compliance with the prohibition of animal testing
Animal testing is prohibited under Article 18 of Cosmetics Regulation No. 1223/2009. Moreover, the Cosmetics Regulation prohibits the marketing of cosmetic products for which the experiments do not respect deadlines explained in the diagram below.
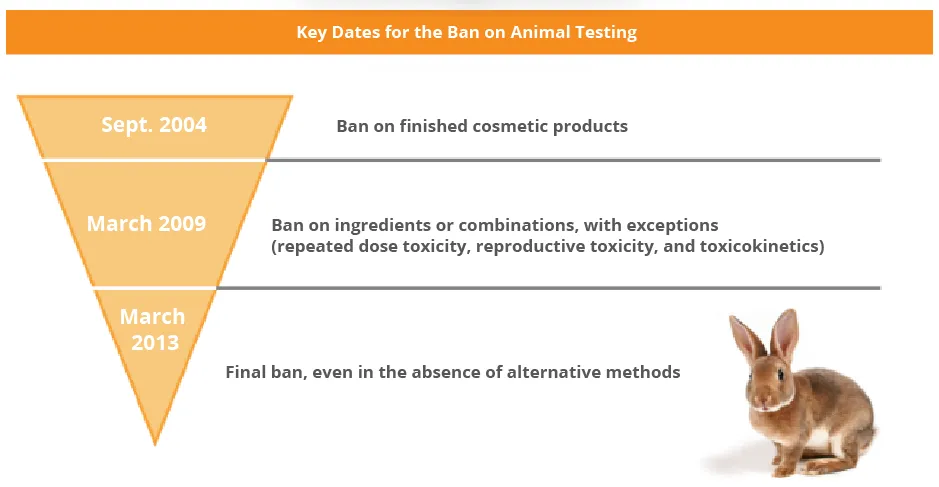
The Responsible Person must verify the correct application of the ban.
Today, the cosmetic industry has developed alternative tools to avoid animal testing, however, animal testing can be tolerated in some very specific instances, contact us for more details on this matter.
6- The Responsible Person ensures the compliance of the packaging and cosmetic label
a) Ensure the compliance of the label of the product (Article 19)
Mandatory requirements on the label:
- Name and address of the Responsible Person
- Country of origin
- Nominal content*
- Date of minimum durability or period-after-opening
- Precautions & warnings*
- Batch number
- Product function*
- Ingredients list (INCI list)
* Translation in the language of the export country is mandatory. Note that Austria, Bulgaria, France, Poland, Portugal and Slovakia request full translation of the label, i.e. even the marketing content and claims.
What labeling symbols should I use?

The Hour-Glass symbol: to illustrate the Date of Minimum Durability (DOMD) when equal or below 30 months. The DOMD is defined by the stability test. You must add the date near the symbol.
If the DOMD exceeds 30 months, the open-jar symbol will indicate the period after opening “PAO” defined by the combination of the stability test and challenge test.

The hand-in-book symbol will indicate to the consumer that a card, tag or leaflet is enclosed with more regulatory information, e.g. the ingredient listing
Labels must contain mandatory indications written indelibly, legibly and visibly.
b) Claims substantiation (Article 20)
The European Cosmetics Regulation aims to protect consumers and end users of cosmetics in the EU. Therefore, it requires that cosmetic claims be truthful and to not mislead consumers.
Therefore, any claim must be substantiated and comply with requirements of Regulation 1223/2009 and Regulation 655/2013 and corresponds to the documents which prove the claimed effect present in the Product Information File. The claims are substantiated by tests and studies included in the PIF.
Article 20 of the Regulation states that: “In the labeling, making available on the market and advertising of cosmetic products, text, names, trademarks, pictures and figurative or other signs shall not be used to imply that these products have characteristics or functions which they do not have.”
c) Indicate the presence of nanomaterial on the pack (Article 16)
In addition to the specific CPNP notification, the Responsible Person must check that the label correctly mentions the presence of nanomaterials by adding the word [nano]. in front of the concerned ingredient, e.g. [nano] titanium dioxide.
For cosmetic products that have been placed on the market before the entry into force of the Cosmetics Regulation in July 2013, Article 16 states: “(…) In addition to the notification under Article 13, cosmetic products containing nanomaterials shall be notified to the Commission by the Responsible Person by electronic means six months prior being placed on the market, except where they have already been placed on the market by the same Responsible Person before 11th January 2013”
7- The Responsible Person proceeds to the CPNP notification
Article 13 of Regulation 1223/2009 requires the RP to forward to the European Commission a number of product information as part of the CPNP portal notification, such as:
- The category of cosmetic product and its name or names, enabling its specific identification
- The name and address of the Responsible Person where the product information file is made readily accessible
- The country of origin in the case of import
- The Member State in which the cosmetic product is to be placed on the market.
- The contact details of a physical person to contact in the case of need
- The presence of substances in the form of nanomaterials
- The name and CAS number (Chemical Abstracts Service or EC number of substances classified as carcinogenic, mutagenic or toxic for reproduction (CMR), of category 1A or 1B, under Part 2 of Annex VI to regulation (EC) No. 1272/2008
- The frame formulation allowing for prompt and appropriate medical treatment in the event of difficulties
Furthermore, Article 13 of the Cosmetics Regulation requires the RP to transmit the original labeling, and packaging photography (if it is legible).
8- Responsible Person and communication
The Responsible Person is the main interlocutor of the EU Member States’ competent authorities. The RP keeps the PIF and must make it accessible to the competent authorities upon their request.
a) Communication of serious undesirable effects (SUE) to the competent authority of the Member State where the SUE was established (Article 23)
Article 23 of the Cosmetics Regulation requires the Responsible Person to immediately notify the competent authorities of the Member State if a serious undesirable effect occurred. The RP must provide:
- All serious undesirable effects which are known to him or which may reasonably be expected to be known to him
- The name of the cosmetic product concerned, enabling its specific identification
- The corrective measures he has taken if any
b) Disclosure of certain information to the public (Article 21)
Article 21 of the EU Cosmetic Regulation 1223/2009, establishes an obligation of transparency to the public. This obligation is also for the Responsible Person.
Thus, the Responsible Person must ensure that the information regarding the qualitative and quantitative composition of the cosmetic product, existing data on undesirable effects and serious undesirable effects is made readily available to the public
Furthermore, the responsible person must ensure that the name and code number of the composition as well as the identity of the supplier for fragrance and aromatic substances are also available to third parties..
EcoMundo can be your Responsible Person
If you’re lost within all of these obligations, it may be good to ask a qualified expert to secure and ensure the marketing of your cosmetic product throughout the EU.
EcoMundo can act as your Responsible Person and guide you during the whole process with a recommended know-how and a trustworthy expertise. Our RP services cover:
- the different obligation and responsibilities of the EU Cosmetic Regulation 1223/2009
- representation before the European Authorities (transmission of all required information relative to your cosmetic product)
- updates thanks to a continuous regulatory watch and cosmetovigilance in order to help you maintain your product on the market
What is a Responsible Person?
The European regulation EC No. 1223/2009 defines the Responsible Person (RP) in the article 4 as a legal or natural person based in the European Union who will act as the unique representative throughout the EU. The RP’s role is to ensure that each cosmetic product personal care product marketed in the EU countries complies with the Cosmetics Regulation 1223/2009 which aims to establish that the cosmetic product is safe for use.
Who can be a Responsible Person?
The Responsible Person may be a manufacturer, importer or distributor, but also a consultant likes EcoMundo. Usually, the one who places the product on the EU market will be the one who will ensure this role unless a third person is designated. Nevertheless you have to keep in mind that only a legal or natural person based in the EU can ensure this role.
A- The manufacturer
The Cosmetics Regulation defines the manufacturer as “any natural or legal person who manufactures a cosmetic product or has such a product designed or manufactured, and markets that cosmetic product under his name or trademark”. »
The manufacturer established within the EU is the Responsible Person for the cosmetic products he manufactures (in the EU) if they are not subject to be exported and reimported into the EU.
The manufacturer may also designate any person established in the community by mandating someone with a written letter. The designated person may accept the role by responding in writing.
B- The importer
Defined by article 2 of the Cosmetics Regulation an importer is “any natural or legal person established within the Community, who places a cosmetic product from a third country on the Community market”. »
C- The distributor
The distributor is defined by the EU Regulation No.1223/2009 as “any natural or legal person in the supply chain, other than the manufacturer or the importer, who makes a cosmetic product available on the community market” »
The distributor is the Responsible Person if the product is placed on the market under his name or trademark or if he modifies a product already on the market and takes the risk to affect the compliance to the Cosmetics Regulation.
D- Any person established in the European Union
The manufacturer or the importer can designate a third person established in the EU to be a Responsible Person.
In this case the only formality required by the Cosmetics Regulation is a written agreement. In other words, you must designate your Responsible Person via a written mandate and the RP must accept his role in writing.
The designated RP can be any legal or natural person who is able to endorse the responsibilities of the RP and comply with the regulatory requirements, e.g. such as a regulatory expert like EcoMundo.
Is it mandatory to have a Responsible Person?
Article 4 of the Cosmetics Regulation establishes that only cosmetic products with an appointed Responsible Person may be placed on the market. In other words: a Responsible Person is essential to market your cosmetics.
Therefore, the Responsible Person has many obligations and responsibilities, especially to:
- Ensure that the cosmetic product placed on the market is safe for human health
- Ensure compliance with Cosmetics Regulation obligations for each cosmetic product placed on the market
What are the obligations of the Responsible Person?
The Responsible Person is in charge of ensuring compliance of the cosmetic product with Regulation 1223/2009 and its amendments.
The RP plays an essential role in the marketing process of a cosmetic product.
If you’re a Responsible Person, you have to endorse all these obligations:
1. The Responsible Person guarantees the safety of the cosmetic product
A- Safety of the product made available on the market (Article 3)
The RP must ensure that the cosmetic product complies with Article 3 of Cosmetics Regulation 1223/2009, that is to say, a “cosmetic product made available on the market shall be safe for human health when used under normal or reasonably foreseeable conditions of use.” ».
B- The cosmetic product safety assessment (Article 10)
The Responsible Person ensures that product safety is assessed on the basis of relevant information, and that the safety assessment of the cosmetic product is established under annex 1 of regulation 1223/2009. The Responsible Person may also incorporate information or indications concerning the security in the product safety report.
Article 10 of Cosmetics Regulation states: “prior to placing a cosmetic product on the market, (the RP must) ensure that the cosmetic product has undergone a safety assessment on the basis of the relevant information and that a cosmetic product safety report is set up in accordance with Annex 1” »
According to this article, the Responsible Person must ensure that:
- The intended use of the cosmetic product and the anticipated systemic exposure to individual ingredients in a final formation are taken into account in the safety assessment
- An appropriate weight-of-evidence approach is used in the safety assessment for reviewing data from all existing sources
- The cosmetic product safety report is kept up to date in view of additional relevant information generated after placing the product on the market.
2- The Responsible Person is in charge of the Product Information File of the cosmetic product
The Product Information File (PIF) must be drafted for each cosmetic product put on the European market.
The Responsible Person guarantees that any product placed on the market is linked to a relevant and complete PIF.
The Responsible Person must keep the Product Information File for a period of 10 years after placing the last batch on the market. The Responsible Person must ensure that the competent authority of the Member State which houses the PIF can access that file easily (electronic or paper), at the location indicated on the label.
3- The Responsible Person ensures the quality of the manufacturing process
a) Good Manufacturing Practice (Article 8)
Good Manufacturing Practices (GMP) are issued from the ISO 22716 guideline published in 2007 which provides guidelines for the production, control, storage and shipment of cosmetic products.
The Responsible Person must ensure the compliance with GMP according to Article 8 of the Cosmetics Regulation. The compliance declaration must be included in the PIF of each cosmetic product.

b) Ensure the compliance of sampling and analysis of cosmetic products (Article 12)
Article 12 of the Cosmetics Regulation states that: “Sampling and analysis of cosmetic products shall be performed in a reliable and reproducible manner.” »
Therefore, the RP must ensure the compliance of the sampling process and the analysis of the cosmetic products in accordance to Article 12 of Cosmetics Regulation 1223/2009 and also make sure these analyses and samples are reliable and reproducible. In addition the Cosmetics Regulation states: “reliability and reproducibility shall be presumed if the method used is in accordance with the relevant harmonized standards, the references of which have been published in the Official Journal of European Union.”.
4- The Responsible Person guarantees the compliance of the cosmetic formula
The Responsible Person, as guarantor of the product’s safety, must verify that the formula meets the Cosmetics Regulation’s requirements. Indeed, some cosmetic ingredients are allowed but some are restricted or prohibited by the Annexes of the Cosmetic Regulation. You must ensure that the product composition placed on the market complies with the restrictions of the Cosmetics regulation.
Moreover, the formula must not contain any prohibited substances unless it is a non-intended trace of a prohibited substance, which is technically unavoidable (articles 14, 15, 16, 17 and 24 of 1223/2009 regulation), i.e. incidental ingredients and impurities.
What are the critical regulatory statuses?
The EU Cosmetic Regulation 1223/2009 features several annexes that list some of the regulated substances susceptible to be harmful to human health. There are three categories of ingredients:
a) Prohibited ingredients
Substances in Annex II:
The prohibited substances are classified in annex II of Cosmetic Regulation 1223/2009.
The CMR substances:

The CMR substances (Carcinogenic, mutagenic or toxic to reproduction) are listed in Annex VI of the CLP Regulation (EC No. 1272/2008), and when they are of 2, 1A, 1B categories: they are prohibited.
They may be authorized under certain conditions:
- If they are compliant with the provisions related to the safety of products, as defined by Regulation (EU) No 178/2002
- In case there is no appropriate substitute substance after the analysis of alternative solutions.
- If a request has been made for a particular use of the products’ category with a determined exposure
- When the substances have been assessed and deemed safe by the SCCS (Scientific Committee on Consumer Safety) for use in cosmetic products (sole condition for category CMR2)
b) Restricted substances
These substances are listed in Annex III of the Cosmetics Regulation, which add up to about 300 substances. These ingredients can be used in cosmetics products only under the conditions described in the annex i.e. product type, purity criteria, percentage of maximal use, etc.
c) Allowed substances
Three functions of ingredients are listed by the Regulation: coloring agents, preservatives and UV filters.
- Allowed coloring agents; they are listed in Annex IV of the Cosmetics Regulation (153 of them)
- Allowed preservatives; they are listed in Annex V (59 of them)
- Allowed UV filters: ; they are listed in Annex VI (29 of them)
These ingredients are authorized if they respect the regulation’s restriction i.e. depending on the type of products in which they are contained, the part of the body that is concerned, as well as the concentration of the ingredient in the product.
The specific case of nanomaterials (Article 16)
If you use a nanomaterial that is not listed in Regulation 1223/2009 annexes, the Responsible Person takes on the responsibility to notify the presence of the nanomaterial at least 6 months before the cosmetic product is placed on the market. The notification must be done via the CPNP portal.
The importance of regulatory watch
To check the regulatory compliance of cosmetic formulas, the Responsible Person must analyze all Annexes and compare the ingredients in the composition of the cosmetic product. It is important to note that this work should be done regularly as theses Annexes are updated several times a year (on average every three months).
Depending on the complexity of your formulas, it may be more relevant for the Responsible Person to be assisted by a software such as COSMETIC Factory
5- The Responsible Person checks the compliance with the prohibition of animal testing
Animal testing is prohibited under Article 18 of Cosmetics Regulation No. 1223/2009. Moreover, the Cosmetics Regulation prohibits the marketing of cosmetic products for which the experiments do not respect deadlines explained in the diagram below.

The Responsible Person must verify the correct application of the ban.
Today, the cosmetic industry has developed alternative tools to avoid animal testing, however, animal testing can be tolerated in some very specific instances, contact us for more details on this matter.
6- The Responsible Person ensures the compliance of the packaging and cosmetic label
a) Ensure the compliance of the label of the product (Article 19)
Mandatory requirements on the label:
- Name and address of the Responsible Person
- Country of origin
- Nominal content*
- Date of minimum durability or period-after-opening
- Precautions & warnings*
- Batch number
- Product function*
- Ingredients list (INCI list)
* Translation in the language of the export country is mandatory. Note that Austria, Bulgaria, France, Poland, Portugal and Slovakia request full translation of the label, i.e. even the marketing content and claims.
What labeling symbols should I use?

The Hour-Glass symbol: to illustrate the Date of Minimum Durability (DOMD) when equal or below 30 months. The DOMD is defined by the stability test. You must add the date near the symbol.

If the DOMD exceeds 30 months, the open-jar symbol will indicate the period after opening “PAO” defined by the combination of the stability test and challenge test.

The hand-in-book symbol will indicate to the consumer that a card, tag or leaflet is enclosed with more regulatory information, e.g. the ingredient listing
Labels must contain mandatory indications written indelibly, legibly and visibly.
b) Claims substantiation (Article 20)
The European Cosmetics Regulation aims to protect consumers and end users of cosmetics in the EU. Therefore, it requires that cosmetic claims be truthful and to not mislead consumers.
Therefore, any claim must be substantiated and comply with requirements of Regulation 1223/2009 and Regulation 655/2013 and corresponds to the documents which prove the claimed effect present in the Product Information File. The claims are substantiated by tests and studies included in the PIF.
Article 20 of the Regulation states that: “In the labeling, making available on the market and advertising of cosmetic products, text, names, trademarks, pictures and figurative or other signs shall not be used to imply that these products have characteristics or functions which they do not have.”
c) Indicate the presence of nanomaterial on the pack (Article 16)
In addition to the specific CPNP notification, the Responsible Person must check that the label correctly mentions the presence of nanomaterials by adding the word [nano]. in front of the concerned ingredient, e.g. [nano] titanium dioxide.
For cosmetic products that have been placed on the market before the entry into force of the Cosmetics Regulation in July 2013, Article 16 states: “(…) In addition to the notification under Article 13, cosmetic products containing nanomaterials shall be notified to the Commission by the Responsible Person by electronic means six months prior being placed on the market, except where they have already been placed on the market by the same Responsible Person before 11th January 2013”
7- The Responsible Person proceeds to the CPNP notification
Article 13 of Regulation 1223/2009 requires the RP to forward to the European Commission a number of product information as part of the CPNP portal notification, such as:
- The category of cosmetic product and its name or names, enabling its specific identification
- The name and address of the Responsible Person where the product information file is made readily accessible
- The country of origin in the case of import
- The Member State in which the cosmetic product is to be placed on the market.
- The contact details of a physical person to contact in the case of need
- The presence of substances in the form of nanomaterials
- The name and CAS number (Chemical Abstracts Service or EC number of substances classified as carcinogenic, mutagenic or toxic for reproduction (CMR), of category 1A or 1B, under Part 2 of Annex VI to regulation (EC) No. 1272/2008
- The frame formulation allowing for prompt and appropriate medical treatment in the event of difficulties
Furthermore, Article 13 of the Cosmetics Regulation requires the RP to transmit the original labeling, and packaging photography (if it is legible).
8- Responsible Person and communication
The Responsible Person is the main interlocutor of the EU Member States’ competent authorities. The RP keeps the PIF and must make it accessible to the competent authorities upon their request.
a) Communication of serious undesirable effects (SUE) to the competent authority of the Member State where the SUE was established (Article 23)
Article 23 of the Cosmetics Regulation requires the Responsible Person to immediately notify the competent authorities of the Member State if a serious undesirable effect occurred. The RP must provide:
- All serious undesirable effects which are known to him or which may reasonably be expected to be known to him
- The name of the cosmetic product concerned, enabling its specific identification
- The corrective measures he has taken if any
b) Disclosure of certain information to the public (Article 21)
Article 21 of the EU Cosmetic Regulation 1223/2009, establishes an obligation of transparency to the public. This obligation is also for the Responsible Person.
Thus, the Responsible Person must ensure that the information regarding the qualitative and quantitative composition of the cosmetic product, existing data on undesirable effects and serious undesirable effects is made readily available to the public
Furthermore, the responsible person must ensure that the name and code number of the composition as well as the identity of the supplier for fragrance and aromatic substances are also available to third parties..
EcoMundo can be your Responsible Person
If you’re lost within all of these obligations, it may be good to ask a qualified expert to secure and ensure the marketing of your cosmetic product throughout the EU.
EcoMundo can act as your Responsible Person and guide you during the whole process with a recommended know-how and a trustworthy expertise. Our RP services cover:
- the different obligation and responsibilities of the EU Cosmetic Regulation 1223/2009
- representation before the European Authorities (transmission of all required information relative to your cosmetic product)
- updates thanks to a continuous regulatory watch and cosmetovigilance in order to help you maintain your product on the market




.png)


