
The Modernization of Cosmetics Regulation Act of 2022 (MoCRA) introduces new standards for cosmetic business registration, product listing, record keeping, adverse event reporting, safety substantiation, GMP, recalls, and more. MoCRA marks the first change to US cosmetic laws at federal level since 1938. Here's our expert analysis of this major regulatory update in the United States to guide you through the MoCRA process.
_
MoCRA's Cosmetic Adverse Events (Sec. 605)
This section stipulates that the Responsible Person shall maintain records related to each report of an adverse event associated with a cosmetic's use in the United States for a period of 6 years. This period is shortened to 3 years for Small Businesses who don't engage in the manufacturing or processing of the cosmetic product.
When a serious adverse event occurs, it must be reported by the Responsible Person within 15 days.
Cosmetic Adverse Events Key Terms & Definitions
_
MoCRA's Good Manufacturing Practices (Sec. 606)
Section 606 of MoCRA requires the FDA to establish Good Manufacturing Practices (GMPs) that are intended to protect the public health and ensure that cosmetic products are not adulterated.
Since MoCRA has only recently been enacted, the FDA has not had the opportunity to create a Cosmetic GMP as required under MoCRA. A Notice of Proposed Rulemaking for the Cosmetic GMP is expected to be published by the FDA by December 2024. The final GMP for cosmetic products facilities will be published by December 2025, including simplified GMP requirements or longer compliance times for smaller businesses.
_
MoCRA Facility Registration and Product Listing (Sec. 607)
Under MoCRA section 607, it's mandatory to register facilities and product listings.
_
Who does MoCRA Facility Registration concern?
Facilities that manufacture or process cosmetics for US distribution are required to be registered under MoCRA Section 607.
Facilities that merely label, package, hold, or distribute cosmetics products, facilities that manufacture ingredients but not final products, and facilities used solely for product research and evaluation purposes are exempt.
_
What are the MoCRA deadlines for Facility Registration?
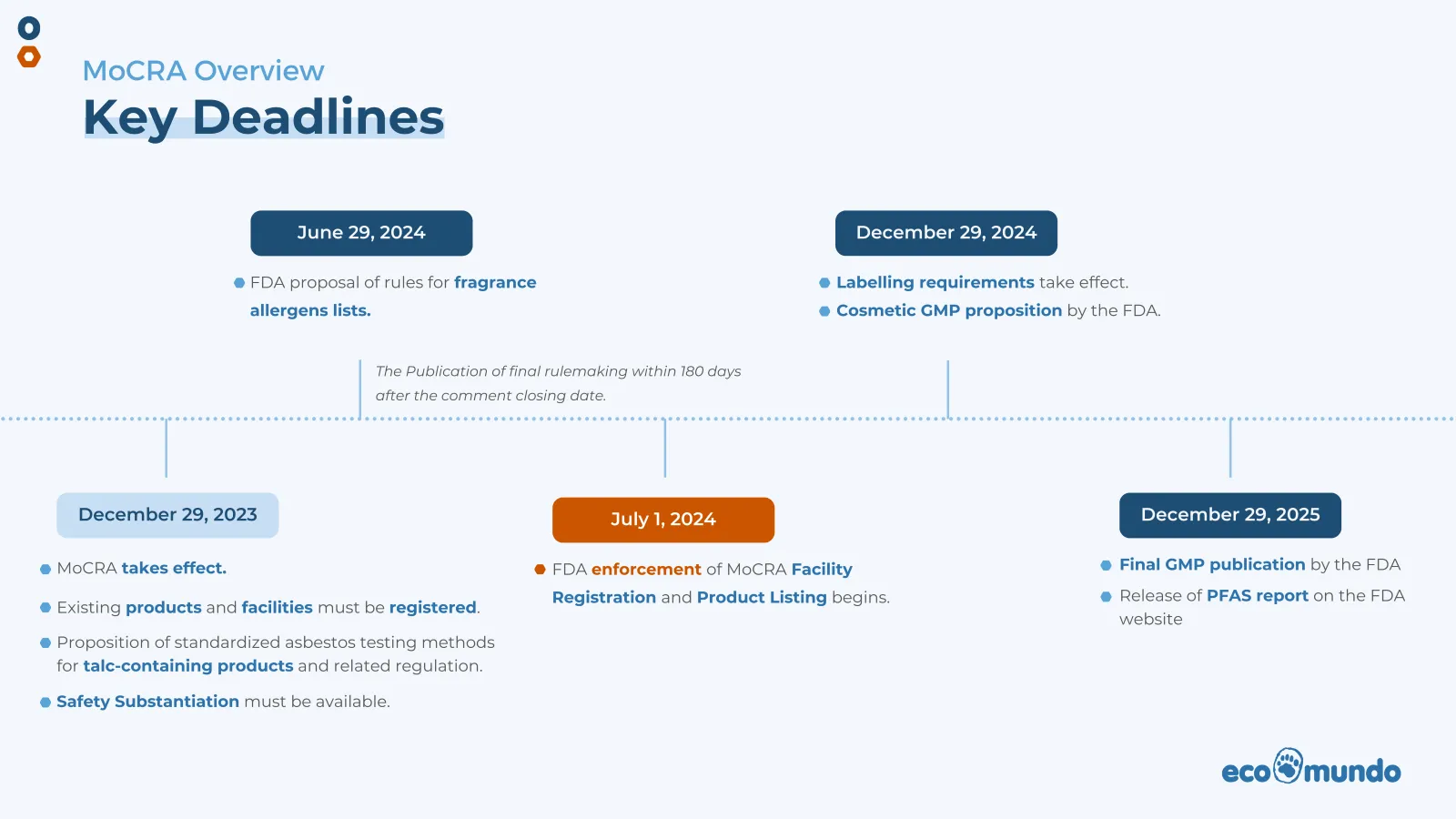
- December 29, 2023 - existing products on the market for safety substantiation reporting.
- July 7, 2024 - product and facility registration (the sooner the better).
New facilities are required to register within 60 days after first engaging in activity.
_
What content should be included in facility registration?
- Facility name, address, email, and phone number.
- Previously assigned facility registration number (if any).
- Cosmetic Brand names manufactured or processed in the facility.
- Cosmetic product category or categories and the responsible person for each product manufactured or processed in the facility.
- United States Agent contact information (for foreign facilities).
_Any changes must be submitted within 60 days and a biennial (every two years) renewal is required.
_
Who does MOCRA's Products Listing concern?
According to Section 607 of MoCRA, the Responsible Person (manufacturer, packer, or distributor of a cosmetic product whose name appears on the label) must submit a products listing.
_
What are the MoCRA deadlines for Products Listing?
- Existing cosmetic products: December 29, 2023.
- New cosmetic products marketed after December 29, 2023: within 120 days.
_
What content should be included in Products Listing?
- Facility registration number(s) where the cosmetic product is manufactured or processed.
- Responsible person's name and contact details.
- Cosmetic product name as it appears on the label.
- Cosmetic product category or categories.
- List of ingredients in the cosmetic product, including any fragrances, flavors, or colors, with each ingredient identified by name, as required below, or by the common or usual name of the ingredient.
- Product listing number (if any).
_
You should note that identical formulations or colors, fragrances and flavor variations may be submitted in a single listing. The RP may submit product listing information as part of a facility registration or separately. Product listing numbers will not be publicly available. Unlike with facility registration, annual updates are required for product listings.
_
_
MoCRA's Safety Substantiation (Sec. 608)
Under MoCRA, the Responsible Person has to ensure, and maintain records supporting that there is adequate substantiation of safety of a cosmetic product.
_
Labeling (Sec. 609)
Under MoCRA, there are a couple of changes to the existing labeling requirements.
- RP information: Cosmetics labels must include a domestic address, domestic phone number, or electronic contact information, which may include a website, through which the responsible person can receive adverse event reports with respect to such cosmetic products.
- Fragrance allergens: For now, a notice of proposed rulemaking establishing a list of fragrance allergens will be published by the FDA no later than June 29, 2024 and a final rulemaking must be published no later than 180 days after the date on which the public comment period on the proposed rulemaking closes. The FDA may establish threshold levels of amount (we may see something similar to the EU allergen disclosure rule).
- Professional Use: The label shall contain a clear and prominent statement that the product shall be administered or used only by licensed professionals.
_
MoCRA's Records (Sec. 610)
If the FDA has a reasonable belief that a cosmetic product is likely to be adulterated, it will be able to have access to and copy all records relating to such cosmetic product, and to any other cosmetic product that is likely to be affected in a similar manner.
Records do not have to include the recipes or formulas for cosmetics, financial data, pricing data, personnel data (other than data as to qualification of technical and professional personnel performing functions subject to this Act), research data (other than safety substantiation data for cosmetic products and their ingredients), or sales data (other than shipment data regarding sales).
_
MoCRA's Mandatory Recall Authority (Sec. 611)
If the FDA determines there is reasonable probability that a cosmetic is adulterated or misbranded and the use or exposure to such cosmetic will cause serious adverse health consequences, the FDA will provide the responsible person the opportunity to cease distribution and recall the product. If the RP refuses, the FDA may require the responsible person by order to immediately cease distribution and recall the product.
_
MoCRA's other notable provisions
- OTC Drug-Cosmetic products are not subject to the above requirements, except for allergen declaration on the label.
- Talc containing products: the FDA shall promulgate proposed regulations to establish and require standardized testing methods for detecting and identifying asbestos in talc-containing cosmetic products not later than December 29, 2023. Final regulations shall be issued not later than 180 days after the date on which the public comment period on the proposed regulations closes.
- PFAS: The FDA shall assess the use of perfluoroalkyl and polyfluoroalkyl substances in cosmetic products and the required scientific evidence regarding the safety of such use in cosmetic products, including any risks associated with such use. A report summarizing the results of the assessment will be published on theFDA�s website by December 29, 2025.
- Animal testing: Should not be used for the purposes of safety testing on cosmetic products and should be phased out with the exception of appropriate allowances. (However, there is no official prohibition of animal testing.)
_
Key MoCRA Terms & Definitions
- Adverse Event: any health-related event associated with the use of a cosmetic product that is adverse.
- Serious Adverse Event: any adverse events that results in death; life-threatening experience; inpatient hospitalization; persistent or significant disability or incapacity; a congenital anomaly or birth defect; an infection; or significant disfigurement (including serious and persistent rashes, second- or third-degree burns, significant hair loss, or persistent or significant alteration of appearance), other than as intended, under conditions of use that are customary or usual; or requires, based on reasonable medical judgment, a medical or surgical intervention to prevent an outcome described above
- Facility: any establishment that manufactures or processes cosmetic products distributed in the United States. It includes filler, but excludes (re)labeler, (re)packer, holder or distributor.
- Responsible Person (RP): manufacturer, packer, or distributor of a cosmetic product whose name appears on the label.
- Small business: an RP, owners and operators of facilities, whose average gross annual sales in the United States of cosmetic products for the previous 3-year period is less than $1,000,000, adjusted for inflation, and who do not engage in the manufacturing or processing of the cosmetic products._
- Professional: an individual who is licensed by an official State authority to practice in the field of cosmetology, nail care, barbering, or esthetics.
- Adequate substantiation: tests or studies, research, analyses, or other evidence or information that is considered, among experts qualified by scientific training and experience to evaluate the safety of cosmetic products and their ingredients, sufficient to support a reasonable certainty that a cosmetic product is safe.
- Safety: is defined as not injurious to users under the conditions of use prescribed in the labeling thereof, or under such conditions of use as are customary or usual.
_
How can EcoMundo help you become MoCRA compliant?
Our regulatory experts are by your side to ensure a smooth adoption of MoCRA standards.
- Safety Substantiation
- Facility Registration
- Product Listing
- US Agent Assistance (for foreign facilities)
- Labeling Review
- Products� Portfolio Management
_
Need help with MoCRA ?
Contact our experts now, they'll be happy to help you!

Helpful resources
- FDA registration portal Cosmetic Direct
- Cosmetics Direct User Guide
- Final industry registration process guidance (new Q&A section on p.18)
- FDA Establishment Identifier (FEI) search portal
The Modernization of Cosmetics Regulation Act of 2022 (MoCRA) introduces new standards for cosmetic business registration, product listing, record keeping, adverse event reporting, safety substantiation, GMP, recalls, and more. MoCRA marks the first change to US cosmetic laws at federal level since 1938. Here's our expert analysis of this major regulatory update in the United States to guide you through the MoCRA process.
_
MoCRA's Cosmetic Adverse Events (Sec. 605)
This section stipulates that the Responsible Person shall maintain records related to each report of an adverse event associated with a cosmetic's use in the United States for a period of 6 years. This period is shortened to 3 years for Small Businesses who don't engage in the manufacturing or processing of the cosmetic product.
When a serious adverse event occurs, it must be reported by the Responsible Person within 15 days.
Cosmetic Adverse Events Key Terms & Definitions
_
MoCRA's Good Manufacturing Practices (Sec. 606)
Section 606 of MoCRA requires the FDA to establish Good Manufacturing Practices (GMPs) that are intended to protect the public health and ensure that cosmetic products are not adulterated.
Since MoCRA has only recently been enacted, the FDA has not had the opportunity to create a Cosmetic GMP as required under MoCRA. A Notice of Proposed Rulemaking for the Cosmetic GMP is expected to be published by the FDA by December 2024. The final GMP for cosmetic products facilities will be published by December 2025, including simplified GMP requirements or longer compliance times for smaller businesses.
_
MoCRA Facility Registration and Product Listing (Sec. 607)
Under MoCRA section 607, it's mandatory to register facilities and product listings.
_
Who does MoCRA Facility Registration concern?
Facilities that manufacture or process cosmetics for US distribution are required to be registered under MoCRA Section 607.
Facilities that merely label, package, hold, or distribute cosmetics products, facilities that manufacture ingredients but not final products, and facilities used solely for product research and evaluation purposes are exempt.
_
What are the MoCRA deadlines for Facility Registration?
- December 29, 2023 - existing products on the market for safety substantiation reporting.
- July 7, 2024 - product and facility registration (the sooner the better).
New facilities are required to register within 60 days after first engaging in activity.
_
What content should be included in facility registration?
- Facility name, address, email, and phone number.
- Previously assigned facility registration number (if any).
- Cosmetic Brand names manufactured or processed in the facility.
- Cosmetic product category or categories and the responsible person for each product manufactured or processed in the facility.
- United States Agent contact information (for foreign facilities).
_Any changes must be submitted within 60 days and a biennial (every two years) renewal is required.
_
Who does MOCRA's Products Listing concern?
According to Section 607 of MoCRA, the Responsible Person (manufacturer, packer, or distributor of a cosmetic product whose name appears on the label) must submit a products listing.
_
What are the MoCRA deadlines for Products Listing?
- Existing cosmetic products: December 29, 2023.
- New cosmetic products marketed after December 29, 2023: within 120 days.
_
What content should be included in Products Listing?
- Facility registration number(s) where the cosmetic product is manufactured or processed.
- Responsible person's name and contact details.
- Cosmetic product name as it appears on the label.
- Cosmetic product category or categories.
- List of ingredients in the cosmetic product, including any fragrances, flavors, or colors, with each ingredient identified by name, as required below, or by the common or usual name of the ingredient.
- Product listing number (if any).
_
You should note that identical formulations or colors, fragrances and flavor variations may be submitted in a single listing. The RP may submit product listing information as part of a facility registration or separately. Product listing numbers will not be publicly available. Unlike with facility registration, annual updates are required for product listings.
_

_
MoCRA's Safety Substantiation (Sec. 608)
Under MoCRA, the Responsible Person has to ensure, and maintain records supporting that there is adequate substantiation of safety of a cosmetic product.
_
Labeling (Sec. 609)
Under MoCRA, there are a couple of changes to the existing labeling requirements.
- RP information: Cosmetics labels must include a domestic address, domestic phone number, or electronic contact information, which may include a website, through which the responsible person can receive adverse event reports with respect to such cosmetic products.
- Fragrance allergens: For now, a notice of proposed rulemaking establishing a list of fragrance allergens will be published by the FDA no later than June 29, 2024 and a final rulemaking must be published no later than 180 days after the date on which the public comment period on the proposed rulemaking closes. The FDA may establish threshold levels of amount (we may see something similar to the EU allergen disclosure rule).
- Professional Use: The label shall contain a clear and prominent statement that the product shall be administered or used only by licensed professionals.
_
MoCRA's Records (Sec. 610)
If the FDA has a reasonable belief that a cosmetic product is likely to be adulterated, it will be able to have access to and copy all records relating to such cosmetic product, and to any other cosmetic product that is likely to be affected in a similar manner.
Records do not have to include the recipes or formulas for cosmetics, financial data, pricing data, personnel data (other than data as to qualification of technical and professional personnel performing functions subject to this Act), research data (other than safety substantiation data for cosmetic products and their ingredients), or sales data (other than shipment data regarding sales).
_
MoCRA's Mandatory Recall Authority (Sec. 611)
If the FDA determines there is reasonable probability that a cosmetic is adulterated or misbranded and the use or exposure to such cosmetic will cause serious adverse health consequences, the FDA will provide the responsible person the opportunity to cease distribution and recall the product. If the RP refuses, the FDA may require the responsible person by order to immediately cease distribution and recall the product.
_
MoCRA's other notable provisions
- OTC Drug-Cosmetic products are not subject to the above requirements, except for allergen declaration on the label.
- Talc containing products: the FDA shall promulgate proposed regulations to establish and require standardized testing methods for detecting and identifying asbestos in talc-containing cosmetic products not later than December 29, 2023. Final regulations shall be issued not later than 180 days after the date on which the public comment period on the proposed regulations closes.
- PFAS: The FDA shall assess the use of perfluoroalkyl and polyfluoroalkyl substances in cosmetic products and the required scientific evidence regarding the safety of such use in cosmetic products, including any risks associated with such use. A report summarizing the results of the assessment will be published on theFDA�s website by December 29, 2025.
- Animal testing: Should not be used for the purposes of safety testing on cosmetic products and should be phased out with the exception of appropriate allowances. (However, there is no official prohibition of animal testing.)
_
Key MoCRA Terms & Definitions
- Adverse Event: any health-related event associated with the use of a cosmetic product that is adverse.
- Serious Adverse Event: any adverse events that results in death; life-threatening experience; inpatient hospitalization; persistent or significant disability or incapacity; a congenital anomaly or birth defect; an infection; or significant disfigurement (including serious and persistent rashes, second- or third-degree burns, significant hair loss, or persistent or significant alteration of appearance), other than as intended, under conditions of use that are customary or usual; or requires, based on reasonable medical judgment, a medical or surgical intervention to prevent an outcome described above
- Facility: any establishment that manufactures or processes cosmetic products distributed in the United States. It includes filler, but excludes (re)labeler, (re)packer, holder or distributor.
- Responsible Person (RP): manufacturer, packer, or distributor of a cosmetic product whose name appears on the label.
- Small business: an RP, owners and operators of facilities, whose average gross annual sales in the United States of cosmetic products for the previous 3-year period is less than $1,000,000, adjusted for inflation, and who do not engage in the manufacturing or processing of the cosmetic products._
- Professional: an individual who is licensed by an official State authority to practice in the field of cosmetology, nail care, barbering, or esthetics.
- Adequate substantiation: tests or studies, research, analyses, or other evidence or information that is considered, among experts qualified by scientific training and experience to evaluate the safety of cosmetic products and their ingredients, sufficient to support a reasonable certainty that a cosmetic product is safe.
- Safety: is defined as not injurious to users under the conditions of use prescribed in the labeling thereof, or under such conditions of use as are customary or usual.
_
How can EcoMundo help you become MoCRA compliant?
Our regulatory experts are by your side to ensure a smooth adoption of MoCRA standards.
- Safety Substantiation
- Facility Registration
- Product Listing
- US Agent Assistance (for foreign facilities)
- Labeling Review
- Products� Portfolio Management
_
Need help with MoCRA ?
Contact our experts now, they'll be happy to help you!

Helpful resources
- FDA registration portal Cosmetic Direct
- Cosmetics Direct User Guide
- Final industry registration process guidance (new Q&A section on p.18)
- FDA Establishment Identifier (FEI) search portal




.png)


