
The Biocidal Products Regulation (EU) 528/2012 (BPR)
_
Since September 1, 2013, the new Biocidal Products Regulation (BPR) EU N�528/2012 replaces the Biocidal Products Directive (BPD) (98/8/EC).
The main objectives are:
- Strengthen the protection of humans and the environment
- Accelerate the evaluation process of active substances (AS)
- To simplify the authorization of biocidal products (BP).
This regulation provides a framework for the definition of biocidal products. Indeed, BPs are products intended to destroy, repel or render harmless harmful organisms. They are active products, defined by an active substance/product type pair.
Biocidal products are divided into four main groups, comprising 22 product types (TP):
- Disinfectants : TP 1 to 5
- Preservatives: TP 6 to 13
- Pest control: TP 14 to 20
- Other biocidal products: TP 21 to 22
The introduction of the BPR has also led to a revision of the procedures for the approval of active substances at EU level and the authorization of biocidal products in the Member States. From now on, only products containing active substances under evaluation or approved by ECHA, for a specific TP, can be placed on the European market.
In this article we will focus on the active substance "ozone generated in situ from oxygen".
_
The current situation - January 2023
_
The active substance 'Ozone generated in situ from oxygen' was submitted for evaluation to ECHA for product types 2, 4, 5 and 11 in September 2016. The dossier was submitted by two different groupings (EurO3zon and European Ozone Trade Association).
The active substance received a positive opinion on December 1, 2021 by the Biocidal Products Committee (BPC) on its approval for the TPs mentioned above (on the dossier submitted by EurO3zon) and a positive opinion on September 26, 2022 for the dossier of the European Ozone Trade Association (EUOTA).
The approval date will be published soon and then voted by the European Commission. This date would be between the end of 2024 and the beginning of 2025, but it remains to be confirmed. Products that use or generate ozone from oxygen can however be placed on the market thanks to the transitional measures according to the national procedures under article 93.
Below is an explanatory diagram of the placing on the market of a biocidal product according to its approval date:
_
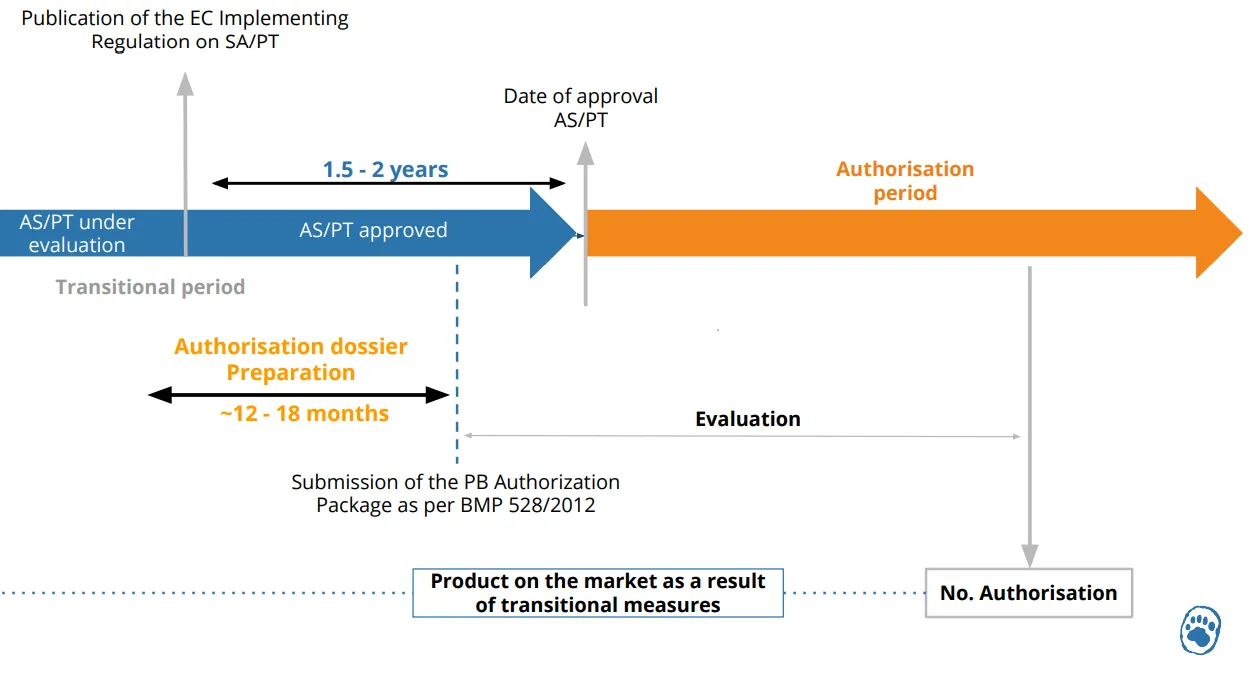
Once the active substance has been approved, a product authorization (PA) dossier will be required to continue selling the biocidal product on the European market
_
What is the next step?
_
After the approval of the active substance, i.e. supposedly at the end of 2024, a product authorization dossier will be required for products generating ozone in situ from oxygen for TP 2, 4, 5 and 11.
The authorization of biocidal products can be issued at national or European level. This strategy is to be evaluated according to several factors, in particular the countries where the product is marketed.
In this article we detail the different types of marketing authorizations, called perennial.
The PA file must contain all the information necessary to evaluate the effectiveness of the product for all the indicated uses and the risks to humans and the environment associated with its use.
In case the product has been notified according to national procedures in the transitional period, and the PA dossier would be submitted before the date of approval of the active substance, then the marketing of the product would not be stopped until the authorization is obtained. If one of the criteria is not met, then the marketing of the product can only start from the date of approval of the PA dossier.
_
EcoMundo Support
At each step we guarantee you a personalized support respecting your deadlines and your confidentiality
1. Strategic diagnosis and cost evaluation
EcoMundo makes an inventory of your obligations and proposes the best strategies adapted to your products and markets, in order to minimize your costs.
2. Risk assessment of the biocidal product
- Consideration of reports from the competent authority on active substances
- Identification of substances of concern
- Exposure modeling
- Risk assessment for human health and the environment
- Follow-up of the approval with the Competent Authorities
3. Preparation of the dossier and submission
EcoMundo supports you from the creation to the submission of your dossier:
- Determine the costs applied to the size of your company
- Realize the " Data gap " of the tox and ecotox data to provide (read-accross, QSAR...)
- Assist in writing the scientific argument
- Assist in the collection of relevant data: analytical, efficacy, toxicology, eco-toxicology, residue analysis etc.
- Fill in the dossier in IUCLID format and generate the dossier
- Submit the dossier via R4BP
4. Intermediary role
EcoMundo defends your interests with :
- ECHA and BPC (Biocidal Product Committee)
- Task forces and consortia
- Data owners to negotiate the costs of LoAs
- Member State evaluation to validate the submission authorization and manage exchanges in case of additional information request.
The Biocidal Products Regulation (EU) 528/2012 (BPR)
_
Since September 1, 2013, the new Biocidal Products Regulation (BPR) EU N�528/2012 replaces the Biocidal Products Directive (BPD) (98/8/EC).
The main objectives are:
- Strengthen the protection of humans and the environment
- Accelerate the evaluation process of active substances (AS)
- To simplify the authorization of biocidal products (BP).
This regulation provides a framework for the definition of biocidal products. Indeed, BPs are products intended to destroy, repel or render harmless harmful organisms. They are active products, defined by an active substance/product type pair.
Biocidal products are divided into four main groups, comprising 22 product types (TP):
- Disinfectants : TP 1 to 5
- Preservatives: TP 6 to 13
- Pest control: TP 14 to 20
- Other biocidal products: TP 21 to 22
The introduction of the BPR has also led to a revision of the procedures for the approval of active substances at EU level and the authorization of biocidal products in the Member States. From now on, only products containing active substances under evaluation or approved by ECHA, for a specific TP, can be placed on the European market.
In this article we will focus on the active substance "ozone generated in situ from oxygen".
_
The current situation - January 2023
_
The active substance 'Ozone generated in situ from oxygen' was submitted for evaluation to ECHA for product types 2, 4, 5 and 11 in September 2016. The dossier was submitted by two different groupings (EurO3zon and European Ozone Trade Association).
The active substance received a positive opinion on December 1, 2021 by the Biocidal Products Committee (BPC) on its approval for the TPs mentioned above (on the dossier submitted by EurO3zon) and a positive opinion on September 26, 2022 for the dossier of the European Ozone Trade Association (EUOTA).
The approval date will be published soon and then voted by the European Commission. This date would be between the end of 2024 and the beginning of 2025, but it remains to be confirmed. Products that use or generate ozone from oxygen can however be placed on the market thanks to the transitional measures according to the national procedures under article 93.
Below is an explanatory diagram of the placing on the market of a biocidal product according to its approval date:
_

Once the active substance has been approved, a product authorization (PA) dossier will be required to continue selling the biocidal product on the European market
_
What is the next step?
_
After the approval of the active substance, i.e. supposedly at the end of 2024, a product authorization dossier will be required for products generating ozone in situ from oxygen for TP 2, 4, 5 and 11.
The authorization of biocidal products can be issued at national or European level. This strategy is to be evaluated according to several factors, in particular the countries where the product is marketed.
In this article we detail the different types of marketing authorizations, called perennial.
The PA file must contain all the information necessary to evaluate the effectiveness of the product for all the indicated uses and the risks to humans and the environment associated with its use.
In case the product has been notified according to national procedures in the transitional period, and the PA dossier would be submitted before the date of approval of the active substance, then the marketing of the product would not be stopped until the authorization is obtained. If one of the criteria is not met, then the marketing of the product can only start from the date of approval of the PA dossier.
_
EcoMundo Support
At each step we guarantee you a personalized support respecting your deadlines and your confidentiality
1. Strategic diagnosis and cost evaluation
EcoMundo makes an inventory of your obligations and proposes the best strategies adapted to your products and markets, in order to minimize your costs.
2. Risk assessment of the biocidal product
- Consideration of reports from the competent authority on active substances
- Identification of substances of concern
- Exposure modeling
- Risk assessment for human health and the environment
- Follow-up of the approval with the Competent Authorities
3. Preparation of the dossier and submission
EcoMundo supports you from the creation to the submission of your dossier:
- Determine the costs applied to the size of your company
- Realize the " Data gap " of the tox and ecotox data to provide (read-accross, QSAR...)
- Assist in writing the scientific argument
- Assist in the collection of relevant data: analytical, efficacy, toxicology, eco-toxicology, residue analysis etc.
- Fill in the dossier in IUCLID format and generate the dossier
- Submit the dossier via R4BP
4. Intermediary role
EcoMundo defends your interests with :
- ECHA and BPC (Biocidal Product Committee)
- Task forces and consortia
- Data owners to negotiate the costs of LoAs
- Member State evaluation to validate the submission authorization and manage exchanges in case of additional information request.







