2024 retrospective and 2025 trends: the dynamics of our Cosmetics & Fragrances customers

EcoMundo's look at the key cosmetics and fragrance markets in 2024
This year we had the pleasure of supporting our Cosmetics & Fragrances customers in many countries on every continent, with a special mention for the three main markets:
United States: Growth Driven by Imports and MoCRA
A global leader in import value, particularly for luxury and specialized products. Imports have seen significant growth, driven by increased demand for international perfume, skincare, and makeup brands.
The MoCRA regulation slightly disrupted stakeholders aiming to maintain or enter the market. However, this did not pose major obstacles for our clients!
We also observed a growing commitment from our clients to comply with state-specific regulations (cosmetics and chemicals). This proactive approach aims to facilitate future alignment between U.S. federal regulations and those of the European Union.
China: A Challenging Yet Promising Market
A highly sought-after market, despite numerous entry barriers. Perfumes, as well as premium and innovative products, continue to find their audience here.
Market access remains complex due to the CSAR regulation. However, for certain product categories, such as perfumes, time-to-market can be reasonable. For example, one of our most successful projects achieved market entry in just six months!
The European Union and the United Kingdom: A Gateway to Other Markets
The EU (and the UK) remains the preferred choice for initial market launches.
Beyond obvious commercial benefits, compliance with these markets’ requirements opens doors to other regions, such as the GCC, ASEAN, and the United Arab Emirates.
With the evolution of related European regulations, particularly those linked to environmental issues (AGEC, microplastics, PFAS, etc.), specialized support is increasingly necessary. EcoMundo is proud to offer its clients comprehensive expertise on the obligations applicable to cosmetics and perfumes, at every stage of their lifecycle, including their components.
The need for systematic, multi-source regulatory monitoring has become a critical concern for formula and packaging manufacturers, brands, and distributors alike.
Throughout the year, EcoMundo kept its clients informed of regulatory updates through webinars and blog articles. Additionally, clients using our services as the EU & UK Responsible Person received tailored regulatory monitoring adapted to their portfolio of substances.
Key Regulatory Developments in 2024
Here is a summary of recent articles published by EcoMundo in 2024 concerning the cosmetics industry. To find out more, click on the title and read the full article.
Directive on Environmental Claims (EU Directive 2024/825):
This directive requires companies to justify their environmental claims to ensure reliability and transparency. It targets claims related to aspects such as waste reduction or carbon footprint, aiming to protect consumers from unfair commercial practices. National transposition is expected by 2026, with strict criteria for labeling and communicating the environmental impacts of products.
Animal Testing Regulations and REACH:
Animal testing bans in the EU and UK remain firm. However, ambiguities persist between cosmetic regulations (CPR) and REACH, prompting debates and calls for clarification. This intersection presents challenges for companies, particularly regarding data requirements for chemical ingredients.
Microplastics in Cosmetic Products:
The revision of Annex XVII of REACH introduces new restrictions on microplastics, notably in makeup and nail products. Companies must adapt formulations to meet transitional requirements and minimize environmental impacts. Obligations include specific labeling, disposal instructions, and detailed reporting to the ECHA.
Environmental Labeling: TRIMAN Logo:
The TRIMAN logo, based on French regulations, is now mandatory for certain products, including cosmetics. This reflects a broader emphasis on environmental compliance across Europe. This change impacts packaging and communication strategies for companies.
Regulatory Changes in Canada:
In 2024, Health Canada introduced new rules aligning some standards with the EU. These changes involve ingredient transparency, restrictions on certain substances, and enhanced cosmetic product safety.
These regulatory developments increase pressure on cosmetic companies to enhance sustainability and compliance. For more information, you can read the full articles directly on EcoMundo’s website.
Conclusion and Future Prospects for the Cosmetics Industry
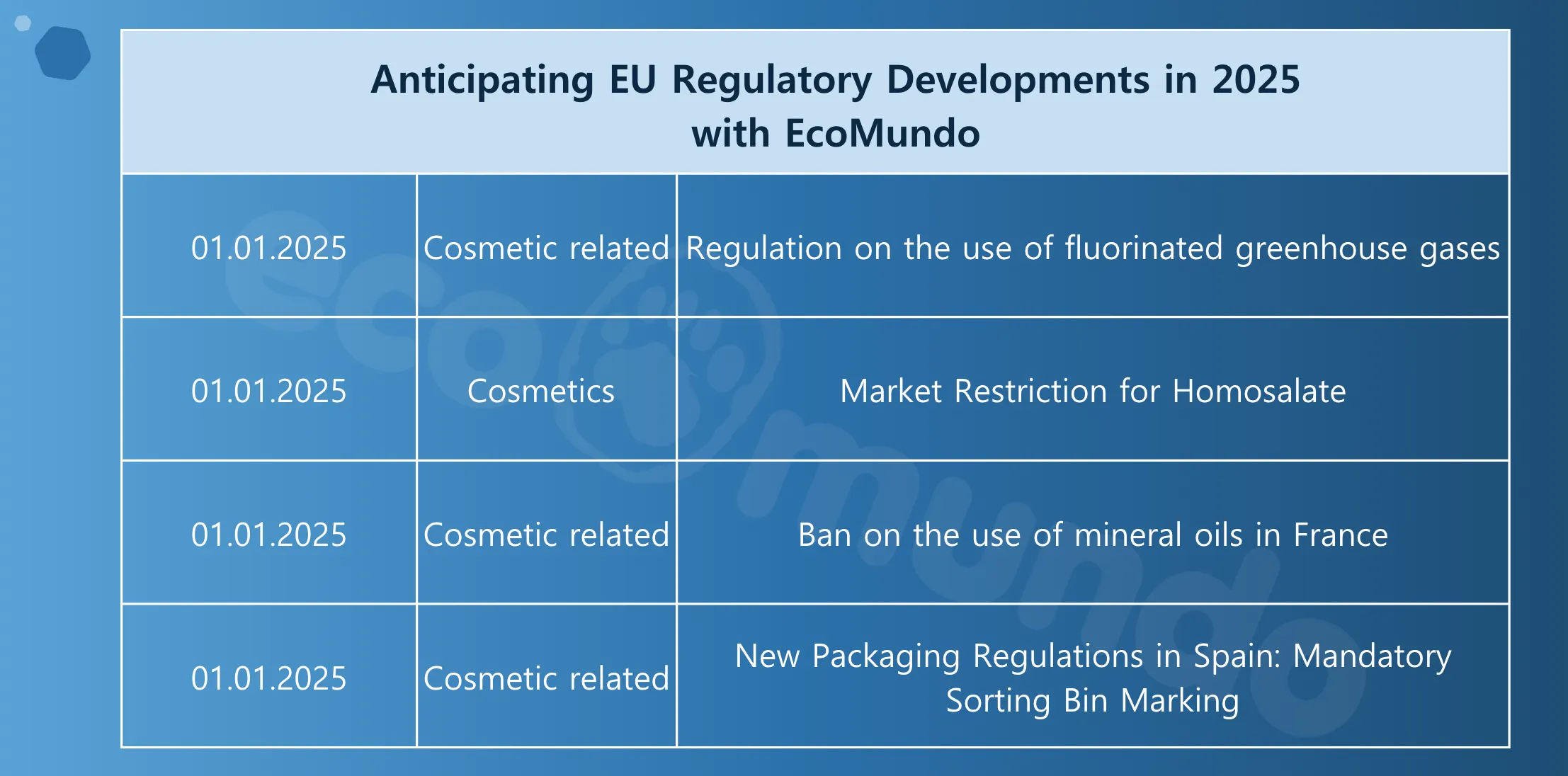
Anticipating regulatory developments in 2025
The beginning of 2025 is set to bring significant regulatory updates. Staying ahead of these changes is essential to remain compliant and competitive in international markets. EcoMundo provides detailed insights and tailored support to secure your market launches.
Here is a preview of the first regulations expected in January 2025 and to benefit from expert guidance or to know more, contact our experts.
EcoMundo's look at the key cosmetics and fragrance markets in 2024
This year we had the pleasure of supporting our Cosmetics & Fragrances customers in many countries on every continent, with a special mention for the three main markets:
United States: Growth Driven by Imports and MoCRA
A global leader in import value, particularly for luxury and specialized products. Imports have seen significant growth, driven by increased demand for international perfume, skincare, and makeup brands.
The MoCRA regulation slightly disrupted stakeholders aiming to maintain or enter the market. However, this did not pose major obstacles for our clients!
We also observed a growing commitment from our clients to comply with state-specific regulations (cosmetics and chemicals). This proactive approach aims to facilitate future alignment between U.S. federal regulations and those of the European Union.
China: A Challenging Yet Promising Market
A highly sought-after market, despite numerous entry barriers. Perfumes, as well as premium and innovative products, continue to find their audience here.
Market access remains complex due to the CSAR regulation. However, for certain product categories, such as perfumes, time-to-market can be reasonable. For example, one of our most successful projects achieved market entry in just six months!
The European Union and the United Kingdom: A Gateway to Other Markets
The EU (and the UK) remains the preferred choice for initial market launches.
Beyond obvious commercial benefits, compliance with these markets’ requirements opens doors to other regions, such as the GCC, ASEAN, and the United Arab Emirates.
With the evolution of related European regulations, particularly those linked to environmental issues (AGEC, microplastics, PFAS, etc.), specialized support is increasingly necessary. EcoMundo is proud to offer its clients comprehensive expertise on the obligations applicable to cosmetics and perfumes, at every stage of their lifecycle, including their components.
The need for systematic, multi-source regulatory monitoring has become a critical concern for formula and packaging manufacturers, brands, and distributors alike.
Throughout the year, EcoMundo kept its clients informed of regulatory updates through webinars and blog articles. Additionally, clients using our services as the EU & UK Responsible Person received tailored regulatory monitoring adapted to their portfolio of substances.
Key Regulatory Developments in 2024
Here is a summary of recent articles published by EcoMundo in 2024 concerning the cosmetics industry. To find out more, click on the title and read the full article.
Directive on Environmental Claims (EU Directive 2024/825):
This directive requires companies to justify their environmental claims to ensure reliability and transparency. It targets claims related to aspects such as waste reduction or carbon footprint, aiming to protect consumers from unfair commercial practices. National transposition is expected by 2026, with strict criteria for labeling and communicating the environmental impacts of products.
Animal Testing Regulations and REACH:
Animal testing bans in the EU and UK remain firm. However, ambiguities persist between cosmetic regulations (CPR) and REACH, prompting debates and calls for clarification. This intersection presents challenges for companies, particularly regarding data requirements for chemical ingredients.
Microplastics in Cosmetic Products:
The revision of Annex XVII of REACH introduces new restrictions on microplastics, notably in makeup and nail products. Companies must adapt formulations to meet transitional requirements and minimize environmental impacts. Obligations include specific labeling, disposal instructions, and detailed reporting to the ECHA.
Environmental Labeling: TRIMAN Logo:
The TRIMAN logo, based on French regulations, is now mandatory for certain products, including cosmetics. This reflects a broader emphasis on environmental compliance across Europe. This change impacts packaging and communication strategies for companies.
Regulatory Changes in Canada:
In 2024, Health Canada introduced new rules aligning some standards with the EU. These changes involve ingredient transparency, restrictions on certain substances, and enhanced cosmetic product safety.
These regulatory developments increase pressure on cosmetic companies to enhance sustainability and compliance. For more information, you can read the full articles directly on EcoMundo’s website.
Conclusion and Future Prospects for the Cosmetics Industry
Anticipating regulatory developments in 2025
The beginning of 2025 is set to bring significant regulatory updates. Staying ahead of these changes is essential to remain compliant and competitive in international markets. EcoMundo provides detailed insights and tailored support to secure your market launches.
Here is a preview of the first regulations expected in January 2025 and to benefit from expert guidance or to know more, contact our experts.





.png)


